The Prognostic Impact of Estimated Creatinine Clearance by Bioelectrical Impedance Analysis in Heart Failure: Comparison of Different eGFR Formulas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Creatinine-Based Formulas for Estimating GFR
- (1)
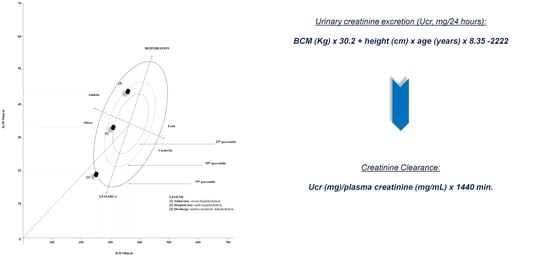
- The Donadio formula was obtained by using tetrapolar impedance plethysmography, which emitted a single alternating sinusoidal current at 50 kHz (CardioEFG, Akern RJL Systems, Florence, Italy). The values of resistance and reactance were measured, and BCM was calculated according to the manufacturer’s equation. Urinary Creatinine Excretion (Ucr) estimation was obtained as follows: Ucr (mg/24 h) = BCM (Kg) × 30.2 + height (cm) × age (years) × 8.35 − 2222, while eGFR was calculated as: Ucr (mg)/plasma creatinine (mg/mL) × 1440 min [17,18];
- (2)
- The Cockroft-Gault formula: (140 − age) × (weight)/(72 × serum creatinine) × 0.85 (if female) [7];
- (3)
- The MDRD-4 formula: 186.3 × creatinine − 1.154 × age − 0.203 × 1.212 (if black) × 0.742 (if female) [8];
- (4)
- The CKD-EPI formula: male: 141 × minimum (creatinine/0.9, 1) − 0.411 × maximum (creatinine/0.9, 1) − 1.209 × 0.993Age × 1.159 (if black); female: 141 × minimum (creatinine/0.7, 1) − 0.329 × maximum (creatinine/0.7, 1) − 1.209 × 0.993Age × 1.018 × 1.159 (if black) [9].
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damman, K.; Valente, M.A.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2013, 35, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of kidney function throughout the heart failure trajectory–A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef]
- Grande, D.; Gioia, M.I.; Terlizzese, P.; Iacoviello, M. Heart failure and kidney disease. Adv. Exp. Med. Biol. 2018, 1067, 219–238. [Google Scholar]
- Bozkurt, B.; Kamat, I.S. Worsening renal function in acute decompensated heart failure: A bad sign, or maybe not? Trans. Am. Clin. Climatol. Assoc. 2019, 130, 41–50. [Google Scholar]
- Kang, J.; Park, J.J.; Cho, Y.; Oh, I.; Park, H.; Lee, S.E.; Kim, M.; Cho, H.; Lee, H.; Choi, J.O.; et al. Predictors and prognostic value of worsening renal function during admission in HFpEF Versus HFrEF: Data from the KorAHF (Korean Acute Heart Failure) registry. J. Am. Heart Assoc. 2018, 7, e007910. [Google Scholar] [CrossRef] [PubMed]
- Grigorian Shamagian, L.; Varela Román, A.; Pedreira Pérez, M.; Gómez Otero, I.; Virgós Lamela, A.; González-Juanatey, J.R. Renal failure is an independent predictor of mortality in hospitalized heart failure patients and is associated with a worse cardiovascular risk profile. Rev. Esp. Cardiol. 2006, 59, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Cockroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Greene, T.; Kusek, J.W.; Beck, G.J. A simplified equation to predict glomerular filtration rate from serum creatinine. J. Am. Soc. Nephrol. 2000, 11, 155A. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Libório, A.; Uchoa, R.; Neto, J.; Valdivia, J.; Daher, E.; Mejia, J. Assessing glomerular filtration rate in patients with severe heart failure: Comparison between creatinine-based formulas. Sao Paulo Med. J. 2012, 130, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, M.A.; Hillege, H.L.; Navis, G.; Voors, A.A.; Dunselman, P.H.; Van Veldhuisen, D.J.; Damman, K. The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur. J. Heart Fail. 2013, 16, 86–94. [Google Scholar] [CrossRef]
- Delanaye, P.; Nellessen, E.; Grosch, S.; DePas, G.; Cavalier, E.; Defraigne, J.-O.; Chapelle, J.-P.; Krzesinski, J.-M.; Lancellotti, P. Creatinine-based formulae for the estimation of glomerular filtration rate in heart transplant recipients. Clin. Transplant. 2006, 20, 596–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smilde, T.D.; van Veldhuisen, D.J.; Navis, G.; Voors, A.A.; Hillege, H.L. Drawbacks and prognostic value of formulas esti-mating renal function in patients with chronic heart failure and systolic dysfunction. Circulation 2006, 114, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Zamora, E.; Lupón, J.; Vila, J.; Urrutia, A.; de Antonio, M.; Sanz, H.; Grau, M.; Ara, J.; Bayés-Genís, A. Estimated glomerular filtration rate and prognosis in heart failure: Value of the modification of diet in renal disease study-4, chronic kidney disease epidemiology collaboration, and cockroft-gault formulas. J. Am. Coll. Cardiol. 2012, 59, 1709–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAlister, F.A.; Ezekowitz, J.; Tarantini, L.; Squire, I.; Komajda, M.; Bayes-Genis, A.; Gotsman, I.; Whalley, G.; Earle, N.; Poppe, K.K.; et al. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) Investigators. Renal dysfunction in pa-tients with heart failure with preserved versus reduced ejection fraction: Impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ. Heart Fail. 2012, 5, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Cerrada, J.C.; Carrasco-Sánchez, F.J.; Perez-Calvo, J.I.; Manzano, L.; Formiga, F.; Bodas, O.A.; Conde, A.; Quiros, R.; Bocanegra, C.P.; Montero-Pérez-Barquero, M.; et al. Prognostic value of glomerular filtration rate estimation equations in acute heart failure with preserved versus reduced ejection fraction. Int. J. Clin. Pract. 2015, 69, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C. Body composition analysis allows the prediction of urinary creatinine excretion and of renal function in chronic kidney disease patients. Nutrients 2017, 9, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donadio, C.; Lucchesi, A.; Tramonti, G.; Bianchi, C. Creatinine clearance can be predicted from plasma creatinine and body composition analysis by means of electrical bioimpedance. Ren. Fail. 1998, 20, 285–293. [Google Scholar] [CrossRef]
- Gardner, R.S.; Chong, K.S.; O’Meara, E.; Jardine, A.; Ford, I.; McDonagh, T.A. Renal dysfunction, as measured by the modi-fication of diet in renal disease equations, and outcome in patients with advanced heart failure. Eur. Heart J. 2007, 28, 3027–3033. [Google Scholar] [CrossRef] [Green Version]
- Singh, T.P.; Givertz, M.M.; Gauvreau, K. Risk stratification for in-hospital mortality after heart transplantation using the modification of diet in renal disease and the chronic kidney disease epidemiology collaboration equations for estimated glo-merular filtration rate. Transplantation 2014, 98, 1000–1006. [Google Scholar] [CrossRef]
- Tancredi, M.; Rosengren, A.; Olsson, M.; Gudbjörnsdottir, S.; Svensson, A.-M.; Haraldsson, B.; Lind, M. The relationship between three eGFR formulas and hospitalization for heart failure in 54,486 individuals with type 2 diabetes. Diabetes/Metab. Res. Rev. 2016, 32, 730–735. [Google Scholar] [CrossRef]
- Skali, H.; Uno, H.; Levey, A.S.; Inker, L.A.; Pfeffer, M.A.; Solomon, S.D. Prognostic assessment of estimated glomerular fil-tration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am. Heart J. 2011, 162, 548–554. [Google Scholar] [CrossRef]
- Oh, J.; Kang, S.-M.; Hong, N.; Youn, J.-C.; Han, S.; Jeon, E.-S.; Cho, M.-C.; Kim, J.-J.; Yoo, B.-S.; Chae, S.C.; et al. KorHF Registry. The CKD-EPI is more accurate in clinical outcome prediction than MDRD equation in acute heart failure: Data from the Korean Heart Failure (KorHF) Registry. Int. J. Cardiol. 2013, 167, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Plischke, M.; Neuhold, S.; Kohl, M.; Heinze, G.; Pacher, R.; Hülsmann, M.; Sunder-Plassmann, G. Renal function in heart failure: A disparity between estimating function and predicting mortality risk. Eur. J. Heart Fail. 2013, 15, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Flores-Blanco, P.J.; Manzano-Fernández, S.; Pérez-Calvo, J.I.; Pastor-Pérez, F.J.; Ruiz, F.J.R.; Carrasco-Sánchez, F.J.; Morales-Rull, J.L.; Figal, D.A.P.; Galisteo-Almeda, L.; Januzzi, J.L. Cystatin C-based CKD-EPI equations and n-terminal pro-b-type natriuretic peptide for predicting outcomes in acutely decompensated heart failure. Clin. Cardiol. 2015, 38, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Fernández, S.; Flores-Blanco, P.J.; Pérez-Calvo, J.I.; Ruiz, F.J.R.; Carrasco-Sánchez, F.J.; Morales-Rull, J.L.; Galisteo-Almeda, L.; Figal, D.A.P.; Valdes, M.; Januzzi, J.L. Comparison of risk prediction with the CKD-EPI and mdrd equations in acute decompensated heart failure. J. Card. Fail. 2013, 19, 583–591. [Google Scholar] [CrossRef]
- Cheang, I.; Liao, S.; Yao, W.; Lu, X.; Gao, R.; Zhou, Y.; Zhang, H.; Li, X. Cystatin C-based CKD-EPI estimated glomerular filtration rate equations as a better strategy for mortality stratification in acute heart failure: A STROBE-compliant prospective observational study. Medicine 2020, 99, e22996. [Google Scholar] [CrossRef]
- Shchekochikhin, D.; Nikiforova, T.; Shilova, A.; Nesterov, A.; Baturina, O.; Gognieva, D.; Kozlovskaya, N.; Syrkin, A.; Kopylov, P. Evaluation of discriminative capacity of two formulas of CKD-EPI to predict complications after the first episode of heart failure with preserved ejection fraction. Int. J. Nephrol. Renov. Dis. 2019, 12, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Zamora, E.; Lupón, J.; De Antonio, M.; Vila-Domènech, J.S.; Peñafiel, J.; Galán, A.; Urrutia, A.; Domingo, M.; Bayes-Genis, A.; Li, Y.C. Long-term prognostic value for patients with chronic heart failure of estimated glomerular filtration rate calculated with the new CKD-EPI equations containing cystatin C. Clin. Chem. 2014, 60, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.Y.; Yang, D.H.; Kim, H.J.; Park, B.E.; Park, Y.J.; Kim, H.N.; Kim, N.K.; Bae, M.H.; Lee, J.H.; Park, H.S.; et al. Prognostic value of cystatin C-derived estimated glomerular filtration rate in patients with acute heart failure. Cardiorenal Med. 2020, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Girerd, N.; Pellicori, P.; Duarte, K.; Girerd, S.; Pfeffer, M.A.; McMurray, J.J.; Pitt, B.; Dickstein, K.; Jacobs, L.; et al. Heart ‘OMics’ in AGEing (HOMAGE) initiative and the High-Risk Myocardial Infarction database initiative. Renal function estimation and Cockroft-Gault formulas for predicting cardiovascular mortality in population-based, cardiovascular risk, heart failure and post-myocardial infarction cohorts: The Heart ‘Omics’ in AGEing (HOMAGE) and the high-risk myocardial infarction database initiatives. BMC Med. 2016, 14, 181. [Google Scholar]
- Scrutinio, D.; Passantino, A.; Santoro, D.; Cacciapaglia, E.; Farinola, G. Prognostic value of formulas estimating excretory renal function in the elderly with systolic heart failure. Age Ageing 2008, 38, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Weidmann, Z.M.; Breidthardt, T.; Twerenbold, R.; Züsli, C.; Nowak, A.; von Eckardstein, A.; Erne, P.; Rentsch, K.; de Oliveira, M.T., Jr.; Gualandro, D.; et al. Prediction of mortality using quantification of renal function in acute heart failure. Int. J. Cardiol. 2015, 201, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; Sanasi, M.; Piscopo, A.; Romito, R.; Valle, R.; Caldarola, P.; et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J. Cardiol. 2020, 75, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Valle, R.; Sanasi, M.; Piscopo, A.; Guida, P.; Mastropasqua, F.; Caldarola, P.; Ciccone, M.M. Serum biochemical determinants of peripheral congestion assessed by bioimpedance vector analysis in acute heart failure. Heart Lung 2019, 48, 395–399. [Google Scholar] [CrossRef]
- Massari, F.; Scicchitano, P.; Ciccone, M.M.; Caldarola, P.; Aspromonte, N.; Iacoviello, M.; Barro, S.; Pantano, I.; Valle, R. Bi-oimpedance vector analysis predicts hospital length of stay in acute heart failure. Nutrition 2019, 61, 56–60. [Google Scholar] [CrossRef]
- Massari, F.; Iacoviello, M.; Scicchitano, P.; Mastropasqua, F.; Guida, P.; Riccioni, G.; Speziale, G.; Caldarola, P.; Ciccone, M.M.; Di Somma, S. Accuracy of bioimpedance vector analysis and brain natriuretic peptide in detection of peripheral edema in acute and chronic heart failure. Heart Lung 2016, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, P.; Massari, F. Bioimpedance vector analysis in the evaluation of congestion in heart failure. Biomark. Med. 2020, 14, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C.; Moriconi, D.; Berta, R.; Anselmino, M. Estimation of urinary creatinine excretion and prediction of renal function in morbidly obese patients: New tools from body composition analysis. Kidney Blood Press. Res. 2017, 42, 629–640. [Google Scholar] [CrossRef]
- Geddes, C.C.; Woo, Y.M.; Brady, S. Glomerular filtration rate--what is the rationale and justification of normalizing GFR for body surface area? Nephrol. Dial. Transpl. 2008, 23, 4–6. [Google Scholar] [CrossRef] [Green Version]
- López-Martínez, M.; Luis-Lima, S.; Morales, E.; Navarro-Díaz, M.; Negrín-Mena, N.; Folgueras, T.; Escamilla, B.; Estupiñán, S.; Delgado-Mallén, P.; Marrero-Miranda, D.; et al. The estimation of GFR and the adjustment for BSA in overweight and obesity: A dreadful combination of two errors. Int. J. Obes. 2019, 44, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Kittiskulnam, P.; Tiskajornsiri, K.; Katavetin, P.; Chaiwatanarat, T.; Eiam-Ong, S.; Praditpornsilpa, K. The failure of glomer-ular filtration rate estimating equations among obese population. PLoS ONE 2020, 15, e0242447. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Egziabher, F.; Brunelle, C.; Thomas, J.; Pelletier, C.C.; Normand, G.; Juillard, L.; Dubourg, L.; Lemoine, S. Estimated glomerular filtration rate bias in participants with severe obesity regardless of deindexation. Obesity 2019, 27, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Pafundi, P.; Garofalo, C.; Galiero, R.; Borrelli, S.; Caturano, A.; Rinaldi, L.; Provenzano, M.; Salvatore, T.; De Nicola, L.; Minutolo, R.; et al. Role of albuminuria in detecting cardio-renal risk and outcome in diabetic subjects. Diagnostics 2021, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021. [Google Scholar] [CrossRef]


| Clinical Characteristics | n = 436 |
|---|---|
| Age, yrs | 75 ± 11 |
| Male, % | 52 |
| BMI, kg/m2 | 28 ± 5 |
| NYHA I-II/III/IV, % | 43/30/27 |
| Peripheral oedema, % | 30 |
| Medical history, % | |
| Coronary artery disease | 30 |
| Diabetes | 24 |
| Atrial fibrillation | 43 |
| PM/ICD | 18 |
| AHF | 42 |
| LVEF, % | 43 ± 12 |
| Preserved LVEF, % | 48 |
| Mid-range LVEF, % | 10 |
| Reduced LVEF, % | 42 |
| Laboratory values | |
| BNP, pg/dL | 510 (199–1100) |
| Hemoglobin, g/dL | 13 ± 2 |
| Uric acid, mg/dL | 6.2 ± 2.1 |
| BUN, mg/dL | 30 ± 17 |
| Creatinine, mg/dL | 1.4 ± 0.9 |
| Sodium, mmol/L | 139 ± 4 |
| Potassium, mmol/L | 4.0 ± 0.6 |
| Chloride, mmol/L | 103 ± 5 |
| Albumin, g/dL | 3.3 ± 0.6 |
| Bioelectrical Impedance Parameters | |
| Resistance/height, Ohm/m | 309 ± 68 |
| Reactance/height, Ohm/m | 27 ± 8 |
| Therapies, % | |
| Furosemide | 69 |
| Beta-blockers | 50 |
| ACE inhibitors/ARBs | 60 |
| MRAs | 69 |
| Digitalis | 21 |
| Ivabradine | 5 |
| eGFR (mL/min/1.73 m2) | Non-Survivors (n = 72) | Survivors (n = 344) | AUC (95% CI) | Cut-Off | Sensitivity | Specificity | PPV | NPV | p |
|---|---|---|---|---|---|---|---|---|---|
| Donadio | 45 ± 28 | 70 ± 35 | 0.72 (0.67–0.76) | <51 | 67 | 69 | 37 | 89 | <0.0001 |
| Cockroft-Gault | 45 ± 20 | 61 ± 28 | 0.75 (0.71–0.79) | <50 | 82 | 57 | 33 | 92 | <0.0001 |
| MDRD-4 | 36 ± 20 | 58 ± 28 | 0.68 (0.64–0.73) | <43 | 54 | 76 | 38 | 86 | <0.0001 |
| CKD-EPI | 38 ± 23 | 58 ± 29 | 0.71 (0.66–0.75) | <41 | 66 | 72 | 39 | 89 | <0.0001 |
| Univariate Cox Regression Analysis | ||
|---|---|---|
| HR (95% CI) | p | |
| Donadio | 0.976 (0.968–0.983) | <0.0001 |
| Cockroft-Gault | 0.962 (0.952–0.972) | <0.0001 |
| MDRD-4 | 0.976 (0.967–0.984) | <0.0001 |
| CKD-EPI | 0.972 (0.963–0.981) | <0.0001 |
| Coronary artery disease | 0.962 (0.610–1.501) | ns |
| Diabetes | 1.081 (0.681–1.722) | ns |
| Atrial fibrillation | 1.422 (0.941–2.15) | ns |
| AHF vs. CHF | 2.721 (1.782–4.12) | <0.0001 |
| NYHA I-II/III/IV | 1.823 (1.45–2.38) | <0.0001 |
| LVEF, % | 0.981 (0.971–1.102) | ns |
| BNP, pg/dL × 100 | 1.040 (1.030–1.051) | <0.0001 |
| Hemoglobin, g/dL | 0.777 (0.701–0.854) | <0.0001 |
| Sodium, mmol/L | 0.925 (0.945–10.67) | ns |
| Adjusted Cox Regression Analysis | |||
|---|---|---|---|
| HR (95% CI) | p | Wald | |
| Donadio | 0.985 (0.977–0.994) | =0.001 | 6.5 |
| BNP, pg/dL × 100 | 1.03 (1.02–1.05) | =0.001 | 15.4 |
| Cockroft-Gault | 0.974 (0.962–0.985) | <0.001 | 17.3 |
| BNP, pg/dL × 100 | 1.02 (1.01–10.4) | =0.002 | 9.4 |
| NYHA I-II/III/IV | 1.363 (1.020–1.816) | =0.036 | 4.4 |
| MDRD-4 | 0.988 (0.979–0.998) | =0.03 | 4.7 |
| BNP, pg/dL × 100 | 1.030 (1.020–1.040) | =0.0002 | 13.8 |
| NYHA I-II/III/IV | 1.336 (1.001–1.783) | <0.04 | 3.9 |
| Hemoglobin, g/dL | 0.886 (0.796–0.987) | =0.03 | 4.8 |
| CKDEPI | 0.984 (0.974–0.994) | =0.003 | 9.4 |
| BNP, pg/dL × 100 | 1.03 (1.010–1.040) | =0.0004 | 12.7 |
| NYHA I-II/III/IV | 1.337 (1.001–1.785) | =0.04 | 17.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicchitano, P.; Iacoviello, M.; Passantino, A.; Guida, P.; De Palo, M.; Piscopo, A.; Gesualdo, M.; Caldarola, P.; Massari, F. The Prognostic Impact of Estimated Creatinine Clearance by Bioelectrical Impedance Analysis in Heart Failure: Comparison of Different eGFR Formulas. Biomedicines 2021, 9, 1307. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9101307
Scicchitano P, Iacoviello M, Passantino A, Guida P, De Palo M, Piscopo A, Gesualdo M, Caldarola P, Massari F. The Prognostic Impact of Estimated Creatinine Clearance by Bioelectrical Impedance Analysis in Heart Failure: Comparison of Different eGFR Formulas. Biomedicines. 2021; 9(10):1307. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9101307
Chicago/Turabian StyleScicchitano, Pietro, Massimo Iacoviello, Andrea Passantino, Piero Guida, Micaela De Palo, Assunta Piscopo, Michele Gesualdo, Pasquale Caldarola, and Francesco Massari. 2021. "The Prognostic Impact of Estimated Creatinine Clearance by Bioelectrical Impedance Analysis in Heart Failure: Comparison of Different eGFR Formulas" Biomedicines 9, no. 10: 1307. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9101307