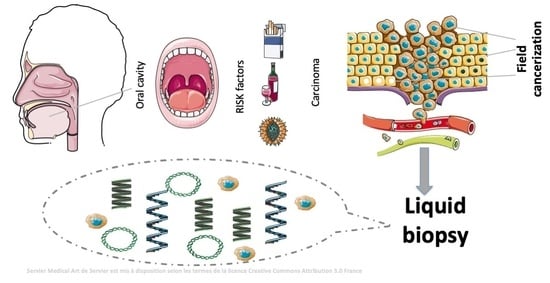
Liquid Biopsy as a Tool for the Characterisation and Early Detection of the Field Cancerization Effect in Patients with Oral Cavity Carcinoma
Abstract
:1. Oral Cavity Carcinoma
2. Field Cancerization Effect in OSCC: Pathophysiology and Associated Problems
2.1. Liquid Biopsy
2.2. ctDNA in Oral Cavity Tumours
2.3. Exosomal RNA
2.4. Exosomes in Blood
2.5. Exosomes in Saliva
2.6. Circulating Tumour Cells (CTCs)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- González-Santiago, S.; Cajal, T.R.; Aguirre, E.; Alés-Martínez, J.E.; Andrés, R.; Balmaña, J.; Graña, B.; Herrero, A.; Llort, G.; González-Del-Alba, A. SEOM clinical guidelines for the treatment of head and neck cancer (2019). Clin. Transl. Oncol. 2020, 22, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.-L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, S.J.; Dahlstrom, K.R.; Peck, B.W.; Caywood, W.; Li, G.; Wei, Q.; Zafereo, M.E.; Sturgis, E.M. Incidence and pattern of second primary malignancies in patients with index oropharyngeal cancers versus index nor opharyngeal head and neck cancers. Cancer 2013, 119, 2593–2601. [Google Scholar] [CrossRef]
- Zhong, L.P.; Zhang, C.P.; Ren, G.X.; Guo, W.; William, W.N., Jr.; Sun, J.; Zhu, H.G.; Tu, W.Y.; Li, J.; Cai, Y.L.; et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin and fluoracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J. Clin. Oncol. 2013, 31, 744–751. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, P.; Bourhis, J.; Lacas, B.; Posner, M.R.; Vermorken, J.B.; Hernandez, J.J.C.; Bourredjem, A.; Calais, G.; Paccagnella, A.; Hitt, R.; et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: An individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J. Clin. Oncol. 2013, 31, 2854–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium: Clinical implication of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef]
- Van Oijen, M.G.; Slootweg, P.J. Oral field cancerization: Carcinogen induced independent events or micrometastatic deposits? Cancer Epidemiol. Prev. Biomark. 2000, 9, 249–256. [Google Scholar]
- Van Oijen, M.G.; Gilsing, M.M.A.; Rijksen, G.; Hordijk, G.J.; Slootweg, P.J. Increased number of proliferating cells in oral epithelium from smokers and ex-smokers. Oral Oncol. 1998, 34, 297–303. [Google Scholar] [CrossRef]
- Mohan, M.; Jagannathan, N. Oral field cancerization: An update on current concepts. Oncol. Rev. 2014, 8, 244. [Google Scholar]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012, 486, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.A., Jr.; Williams, R.T.; Wu, J.; Kinde, I.; Hecht, J.R.; Berlin, J.; Allen, B.; Bozic, I.; Reiter, J.G.; Nowak, M.A.; et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012, 486, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-L.; Tong, R.-Z.; Zhang, Y.; Hu, B.-B.; Zheng, K.; Ding, Z.-Y.; Peng, F.; Gong, Y.-L.; Liu, Y.-M.; Lu, Y. Conventional real-time PCR-based detection of T790M using tumor tissue or blood in patients with EGFR TKI-resistant NSCLC. OncoTargets Ther. 2017, 10, 3307–3312. [Google Scholar] [CrossRef] [Green Version]
- Vidal, J.; Muinelo, L.; Dalmases, A.; Jones, F.; Edelstein, D.; Iglesias, M.; Orrillo, M.; Abalo, A.; Rodríguez, C.; Brozos, E.; et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 2017, 28, 1325–1332. [Google Scholar] [CrossRef]
- Mandel, P. [Nuclear Acids In Human Blood Plasma]. CR Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Chan, K.A.; Jiang, P.; Zheng, Y.W.; Liao, G.J.; Sun, H.; Wong, J.; Siu, S.S.N.; Chan, W.C.; Chan, S.L.; Chan, A.T.; et al. Cancer genome scanning in plasma: Detection of tu-mor-associated copy number aberrations, single-nucleotide variants, and tumoral heterogeneity by massively parallel se-quencing. Clin. Chem. 2013, 59, 211–224. Available online: https://pubmed.ncbi.nlm.nih.gov/23065472/ (accessed on 24 June 2021). [CrossRef] [Green Version]
- Bidard, F.C.; Weigelt, B.; Reis-Filho, J.S. Going with the flow: From circulating tumor cells to DNA. Sci. Transl. Med. 2013, 5, 207ps14. Available online: https://pubmed.ncbi.nlm.nih.gov/24132635/ (accessed on 24 June 2021). [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. Available online: https://pubmed.ncbi.nlm.nih.gov/23836314/ (accessed on 24 June 2021). [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. Available online: https://pubmed.ncbi.nlm.nih.gov/24449238/ (accessed on 24 June 2021). [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.B.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. Available online: https://pubmed.ncbi.nlm.nih.gov/21562580/ (accessed on 24 June 2021). [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. Available online: https://pubmed.ncbi.nlm.nih.gov/25631445/ (accessed on 24 June 2021). [CrossRef] [Green Version]
- Lebofsky, R.; Decraene, C.; Bernard, V.; Kamal, M.; Blin, A.; Leroy, Q.; Frio, T.R.; Pierron, G.; Callens, C.; Bieche, I.; et al. Circulating tumor DNA as a non-invasive substitute to metastasis biopsy for tumor genotyping and personalized medicine in a prospective trial across all tumor types. Mol. Oncol. 2015, 9, 783–790. [Google Scholar] [CrossRef]
- Noronha Nunes, D.; Paulo Kowalski, L.; Simpson, A.J. Circulating tumor-derived DNA may permit the early diagnosis of head and neck squamous cell carcinomas. Int. J. Cancer 2001, 92, 214–219. [Google Scholar] [CrossRef]
- Hamana, K.; Uzawa, K.; Ogawara, K.; Shiiba, M.; Bukawa, H.; Yokoe, H.; Tanzawa, H. Monitoring of circulating tumour-associated DNA as a prognostic tool for oral squamous cell carcinoma. Br. J. Cancer 2005, 92, 2181–2184. [Google Scholar] [CrossRef] [Green Version]
- Kakimoto, Y.; Yamamoto, N.; Shibahara, T. Microsatellite analysis of serum DNA in patients with oral squamous cell carcinoma. Oncol. Rep. 1994, 20, 1195–1200. [Google Scholar] [CrossRef]
- Shukla, D.; Kale, A.D.; Hallikerimath, S.; Yerramalla, V.; Subbiah, V. Can quantifying free-circulating DNA be a diagnostic and prognostic marker in oral epithelial dysplasia and oral squamous cell carcinoma? J. Oral Maxillofac. Surg. 2013, 71, 414–418. [Google Scholar] [CrossRef]
- Wang, Y.; Springer, S.; Mulvey, C.L.; Silliman, N.; Schaefer, J.; Sausen, M.; James, N.; Rettig, E.M.; Guo, T.; Pickering, C.; et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci. Transl. Med. 2015, 7, 293ra104. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, A.M.; Rutkowski, T.; Fiszer-Kierzkowska, A.; Małusecka, E.; Składowski, K. Assessment of the total cfDNA and HPV16/18 detection in plasma samples of head and neck squamous cell carcinoma patients. Oral Oncol. 2016, 54, 36–41. [Google Scholar] [CrossRef]
- Perdomo, S.; Avogbe, P.H.; Foll, M.; Abedi-Ardekani, B.; Facciolla, V.L.; Anantharaman, D.; Chopard, P.; Le Calvez-Kelm, F.; Vilensky, M.; Polesel, J.; et al. Circulating tumor DNA detection in head and neck cancer: Evaluation of two different detection approaches. Oncotarget 2017, 8, 72621–72632. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, A.; Hariharan, A.K.; Hasina, R.; Nair, J.R.; Katragadda, S.; Irusappan, S.; Ravichandran, A.; Veeramachaneni, V.; Bettadapura, R.; Bhati, M.; et al. Ultrasensitive detection of tumor-specific mutations in saliva of patients with oral cavity squamous cell carcinoma. Cancer 2021, 127, 1576–1589. [Google Scholar] [CrossRef]
- Mazumder, S.; Datta, S.; Ray, J.G.; Chaudhuri, K.; Chatterjee, R. Liquid biopsy: miRNA as a potential biomarker in oral cancer. Cancer Epidemiol. 2019, 58, 137–145. Available online: https://pubmed.ncbi.nlm.nih.gov/30579238/ (accessed on 24 June 2021). [CrossRef]
- Rapado-González, O.; Martínez-Reglero, C.; Salgado-Barreira, A.; López-López, R.; Suárez-Cunqueiro, M.; Muinelo-Romay, L. miRNAs in liquid biopsy for oral squamous cell carcinoma diagnosis: Systematic review and meta-analysis. Oral Oncol. 2019, 99, 104465. [Google Scholar] [CrossRef]
- Menini, M.; De Giovanni, E.; Bagnasco, F.; Delucchi, F.; Pera, F.; Baldi, D.; Pesce, P. Salivary micro-RNA and oral squamous cell carcinoma: A systematic review. J. Pers. Med. 2021, 11, 101. [Google Scholar] [CrossRef]
- Rapado-González, Ó.; López-López, R.; López-Cedrún, J.L.; Triana-Martínez, G.; Muinelo-Romay, L.; Suárez-Cunqueiro, M.M. Cell-free microRNAs as potential oral cancer biomarkers: From diagnosis to therapy. Cells 2019, 8, 1653. [Google Scholar] [CrossRef] [Green Version]
- Rabinowits, G.; Bowden, M.; Flores, L.M.; Verselis, S.; Vergara, V.; Jo, V.Y.; Chau, N.; Lorch, J.; Hammerman, P.S.; Thomas, T.; et al. Comparative analysis of microRNA expression among benign and malignant tongue tissue and plasma of patients with tongue cancer. Front. Oncol. 2017, 7, 191. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, C.; Wang, S.; Wang, Z.; Jiang, J.; Wang, W.; Li, X.; Chen, J.; Liu, K.; Li, C.; et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016, 76, 1770–1780. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Patel, S.; Modi, B.; Shah, F.; Rawal, R. Uncovering the potential of CD44v/SYNE1/miR34a axis in salivary fluids of oral cancer patients. J. Oral Pathol. Med. 2018, 47, 345–352. [Google Scholar] [CrossRef]
- He, L.; Ping, F.; Fan, Z.; Zhang, C.; Deng, M.; Cheng, B.; Xia, J. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharmacother. 2020, 121, 109553. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Trachtenberg, A.; Kuo, W.; Cheng, Y. Genomewide study of salivary microRNAs for detection of oral cancer. J. Dent. Res. 2014, 93, 86S–93S. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. Available online: https://pubmed.ncbi.nlm.nih.gov/21560073/ (accessed on 24 June 2021). [CrossRef] [Green Version]
- O’Loughlin, A.J.; Woffindale, C.A.; Wood, M.J.A. Exosomes and the emerging field of exosome-based gene therapy. Curr. Gene Ther. 2012, 12, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iero, M.; Valenti, R.; Huber, V.; Filipazzi, P.; Parmiani, G.; Fais, S.; Rivoltini, L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008, 15, 80–88. Available online: https://pubmed.ncbi.nlm.nih.gov/17932500/ (accessed on 24 June 2021). [CrossRef]
- Guo, W.; Gao, Y.; Li, N.; Shao, F.; Wang, C.; Wang, P.; Yang, Z.; Li, R.; He, J. Exosomes: New players in cancer. Oncol. Rep. 2017, 38, 665–675. Available online: https://pubmed.ncbi.nlm.nih.gov/28627679/ (accessed on 24 June 2021). [CrossRef] [Green Version]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. The potential of tumor-derived exosomes for noninvasive cancer monitoring: An update. Expert Rev. Mol. Diagn. 2018, 18, 1029–1040. Available online: https://pubmed.ncbi.nlm.nih.gov/30406709/ (accessed on 24 June 2021). [CrossRef]
- Zlotogorski-Hurvitz, A.; Dayan, D.; Chaushu, G.; Salo, T.; Vered, M. Morphological and molecular features of oral fluid-derived exosomes: Oral cancer patients versus healthy individuals. J. Cancer Res. Clin. Oncol. 2016, 142, 101–110. [Google Scholar] [CrossRef]
- Kawakubo-Yasukochi, T.; Morioka, M.; Hazekawa, M.; Yasukochi, A.; Nishinakagawa, T.; Ono, K.; Kawano, S.; Nakamura, S.; Nakashima, M. miR-200c-3p spreads in-vasive capacity in human oral squamous cell carcinoma microenvironment. Mol. Carcinog. 2018, 57, 295–302. Available online: https://pubmed.ncbi.nlm.nih.gov/28981169/ (accessed on 24 June 2021). [CrossRef] [PubMed]
- Abels, E.R.; Breakefield, X.O. Introduction to extracellular vesicles: Biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Dickman, C.T.; Lawson, J.; Jabalee, J.; MacLellan, S.A.; LePard, N.E.; Bennewith, K.L.; Garnis, C. Selective extracellular vesicle exclusion of miR-142-3p by oral cancer cells promotes both internal and extracellular malignant phenotypes. Oncotarget 2017, 8, 15252–15266. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.P.; Xu, K.; Cui, J.; Yuan, D.Y.; Zou, B.; Li, J.; Liu, J.L.; Li, K.Y.; Meng, Z.; Zhang, B. Cancer-associated fibroblast-derived exosomal miR-382-5p promotes the migration and invasion of oral squamous cell carcinoma. Oncol. Rep. 2019, 42, 1319–1328. Available online: https://pubmed.ncbi.nlm.nih.gov/31364748/ (accessed on 24 June 2021). [CrossRef] [PubMed]
- Xu, H.; Yang, Y.; Zhao, H.; Yang, X.; Luo, Y.; Ren, Y.; Liu, W.; Li, N. Serum miR-483-5p: A novel diagnostic and prognostic bi-omarker for patients with oral squamous cell carcinoma. Tumour Biol. 2016, 37, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, S.R.; Pérez-Sayans, M.; Fais, S.; Logozzi, M.; Torreira, M.G.; García, A.G. A pilot clinical study on the prognostic relevance of plasmatic exosome levels in oral squamous cell carcinoma patients. Cancers 2019, 11, 429. Available online: https://pubmed.ncbi.nlm.nih.gov/30917536/ (accessed on 24 June 2021). [CrossRef] [PubMed] [Green Version]
- Pedersen, N.J.; Jensen, D.H.; Lelkaitis, G.; Kiss, K.; Charabi, B.W.; Ullum, H.; Specht, L.; Schmidt, A.Y.; Nielsen, F.C.; von Buchwald, C. MicroRNA-based classifiers for diagnosis of oral cavity squamous cell carcinoma in tissue and plasma. Oral Oncol. 2018, 83, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, Q.; Liang, J.; Li, W.; Su, Q.; Chen, Z.; Wan, Q.; Zhou, X.; Cao, L.; Sun, J.; et al. miR-31- 5p is a potential circulating biomarker and therapeutic target for oral cancer. Mol. Ther.-Nucleic Acids 2019, 16, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-C.; Liu, C.-J.; Lin, J.-A.; Chiang, W.-F.; Hung, P.-S.; Chang, K.-W. miR-24 up-regulation in oral carcinoma: Positive association from clinical and in vitro analysis. Oral Oncol. 2010, 46, 204–208. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, C.; Chi, J.; Li, J.; Peng, C.; Yun, X.; Li, D.; Yu, Y.; Li, Y.; Gao, M.; et al. miR-24 promotes the proliferation, migration and invasion in human tongue squamous cell carcinoma by targeting FBXW7. Oncol. Rep. 2016, 36, 1143–1149. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, H.; Sho, R.; Takeda, Y.; Zhang, X.; Yoshida, Y.; Narimatsu, H.; Otani, K.; Ishikawa, S.; Fukao, A.; Asao, H.; et al. Cir-culating miR-223 in oral cancer: Its potential as a novel diagnostic biomarker and therapeutic target. PLoS ONE 2016, 11, e0159693. [Google Scholar] [CrossRef]
- Aiello, N.; Maddipati, R.; Norgard, R.J.; Balli, D.; Li, J.; Yuan, S.; Yamazoe, T.; Black, T.; Sahmoud, A.; Furth, E.E.; et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 2018, 45, 681–695.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, R.L. Immunology and immunotherapy of head and neck cancer. J. Clin. Oncol. 2015, 33, 3293–3304. Available online: https://pubmed.ncbi.nlm.nih.gov/26351330/ (accessed on 24 June 2021). [CrossRef] [PubMed]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical significance of PD-L1 þ exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018, 24, 896–905. Available online: https://pubmed.ncbi.nlm.nih.gov/29233903/ (accessed on 24 June 2021). [CrossRef] [Green Version]
- Schulz, B.L.; Cooper-White, J.; Punyadeera, C.K. Saliva proteome research: Current status and future outlook. Crit. Rev. Biotechnol. 2013, 33, 246–259. Available online: https://pubmed.ncbi.nlm.nih.gov/22612344/ (accessed on 24 June 2021). [CrossRef]
- Gai, C.; Camussi, F.; Broccoletti, R.; Gambino, A.; Cabras, M.; Molinaro, L.; Carossa, S.; Camussi, G.; Arduino, P.G. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, Y.-X.; Yang, X.; Jiang, L.; Zhou, Z.-J.; Zhu, Y.-Q. Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer 2013, 13, 129. [Google Scholar] [CrossRef] [Green Version]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, Y.; Tsujimoto, M.; Yanoshita, R. Next-generation sequencing of protein-coding and long non-protein-coding RNAs in two types of exosomes derived from human whole saliva. Biol. Pharm. Bull. 2016, 39, 1496–1507. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, Y.; Kanai-Azuma, M.; Akimoto, Y.; Kawakami, H.; Yanoshita, R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol. Pharm. Bull. 2008, 31, 1059–1062. [Google Scholar] [CrossRef] [Green Version]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary bi-omarkers. Oncotarget 2017, 8, 82459–82474. Available online: https://pubmed.ncbi.nlm.nih.gov/29137278/ (accessed on 24 June 2021). [CrossRef] [PubMed] [Green Version]
- Patel, S.; Shah, K.; Mirza, S.; Shah, K.; Rawal, R. Circulating tumor stem like cells in oral squamous cell carcinoma: An unresolved paradox. Oral Oncol. 2016, 62, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Chang, Y.-C. Strategies for isolation and molecular profiling of circulating tumor cells. In Results and Problems in Cell Differentiation; Springer: New York, NY, USA, 2017; Volume 994, pp. 43–66. [Google Scholar]
- Inhestern, J.; Oertel, K.; Stemmann, V.; Schmalenberg, H.; Dietz, A.; Rotter, N.; Veit, J.; Görner, M.; Sudhoff, H.; Junghanß, C.; et al. Prognostic role of circulating tumor cells during induction chemotherapy followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer. PLoS ONE 2015, 10, e0132901. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Hatori, M.; Kinugasa, Y.; Irie, T.; Tachikawa, T.; Nagumo, M. Comparison of the expression profile of metasta-sis-associated genes between primary and circulating cancer cells in oral squamous cell carcinoma. Anti. Cancer Res. 2003, 23, 1425–1431. [Google Scholar]
- Gröbe, A.; Blessmann, M.; Hanken, H.; Friedrich, R.E.; Schön, G.; Wikner, J.; Effenberger, K.E.; Kluwe, L.; Heiland, M.; Pantel, K.; et al. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin. Cancer Res. 2014, 20, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Costa, J.P.; De Carvalho, A.F.; da Silveira, G.G.; Amaya, P.; Wu, Y.; Park, K.-J.J.; Gigliola, M.P.; Lustberg, M.; Buim, M.E.C.; Ferreira, E.; et al. Gene expression patterns through oral squamous cell carcinoma development: PD-L1 expression in primary tumor and circulating tumor cells. Oncotarget 2015, 6, 20902–20920. [Google Scholar] [CrossRef] [Green Version]
- Strati, A.; Koutsodontis, G.; Papaxoinis, G.; Angelidis, I.; Zavridou, M.; Economopoulou, P.; Kotsantis, I.; Avgeris, M.; Mazel, M.; Perisanidis, C.; et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1923–1933. [Google Scholar] [CrossRef]
| Authors, Year | OSCCs (n) | Other Tissue (n) | Technique/Detection | Results | Discussion/Conclusion |
|---|---|---|---|---|---|
| Nunes et al. (2001) [26] | 46 | 45 | Eight microsatellite markers in tissue and serum (cfDNA) | 58% had microsatellite alterations and 17 patients had the same profile in plasma. | Early detection. |
| Hamana et al. (2005) [27] | 64 | None | Nine microsatellite markers in tissue and serum (pre, immediately post-surgery and 4 weeks after surgery | Allelic imbalance patterns in serum were associated with the presence of allelic imbalance in paired tumour tissue. Patients with allelic imbalance four weeks after surgery developed metastases | Microsatellite analysis could help assess the risk of recurrence. |
| Kakimoto et al. (2008) [28] | 20 | None | Nine microsatellite markers in tissue and serum (one month before and after surgery) | Allelic imbalance in ctDNA was observed in blood in 90% of patients | Microsatellite analysis could help assess the risk of recurrence. |
| Shukla et al. (2013) [29] | 150 OSCCs150 post-treatment OSCCs | 90 potentially malignant lesions | Quantity of cfDNA in plasma by spectrophotometry | No differences | Rich lymphatic drainage of the oral mucosa prevents it from entering the bloodstream. |
| Wang et al. (2015) [30] | 15 | 78 | Pre and post-treatment (9) samples of blood and saliva to detect ctDNA and mutations by multiplex PCR. | - ctDNA in blood and saliva was found in 96% of 47 patients. - ctDNA was present in 100% of saliva and blood specimens of OSCCs. - After treatment, four patients with ctDNA underwent recurrence. | Utility in monitoring. Saliva provides a more sensitive predictor of early-stage disease than plasma |
| Mazurek et al. (2016) [31] | Unknown | 200 | HPV16/18, KRAS and EGFR by q-PCR | 14% were HPV16+ Neither EGFR and KRAS were detected. HPV was found in 86% of plasma and 40% of saliva specimens. | HPV cfDNA could be used for the early detection and monitoring of HPV+. |
| Perdomo et al. (2017) [32] | 41 | Other head and neck cancer | Approaches: (a) Mutations in ctDNA of five genes identified in tissue and plasma (b) TP53 mutation analysed in tissue, plasma and oral rinses. | (a) 18 mutations in 42% of patients (b) 36%, 3% and 26% of TP53 mutation (tissue, plasma and oral rinses) | Concordance of mutation detection was low between tumour tissue, oral rinses and plasma. |
| Shanmugam et al. (2021) [33] | 121 | None | Next generation sequence (NGS) with tissue and saliva specimens (before surgery) | In 95.87% of cases, at least one somatic variant was identified. The most prevalent mutated genes were TP53, FAT1, CDKN2A and NOTCH1 (the same as in tumour tissue). Concordance between mutations in tumour and saliva was >97% but less in early-stage disease. | Sequencing platforms could be used to screen high-risk individuals, facilitating early detection and monitoring. |
| Liquid Biopsy Techniques | Diagnosis/Prognosis | References |
|---|---|---|
| ctDNA | Diagnosis > Prognosis | [26,32,33] |
| miRNA | Diagnosis > prognosis | [35,36] |
| Exosomes | Diagnosis | [50,71] |
| CTC | Prognosis | [74,75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Ruiz, E.; Gutiérrez, V.; Muñoz, M.; Oliver, J.; Sánchez, M.; Gálvez-Carvajal, L.; Rueda-Domínguez, A.; Barragán, I. Liquid Biopsy as a Tool for the Characterisation and Early Detection of the Field Cancerization Effect in Patients with Oral Cavity Carcinoma. Biomedicines 2021, 9, 1478. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9101478
Pérez-Ruiz E, Gutiérrez V, Muñoz M, Oliver J, Sánchez M, Gálvez-Carvajal L, Rueda-Domínguez A, Barragán I. Liquid Biopsy as a Tool for the Characterisation and Early Detection of the Field Cancerization Effect in Patients with Oral Cavity Carcinoma. Biomedicines. 2021; 9(10):1478. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9101478
Chicago/Turabian StylePérez-Ruiz, Elisabeth, Vanesa Gutiérrez, Marta Muñoz, Javier Oliver, Marta Sánchez, Laura Gálvez-Carvajal, Antonio Rueda-Domínguez, and Isabel Barragán. 2021. "Liquid Biopsy as a Tool for the Characterisation and Early Detection of the Field Cancerization Effect in Patients with Oral Cavity Carcinoma" Biomedicines 9, no. 10: 1478. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9101478