The Differential Effects of HDL Subpopulations on Lipoprotein Lipase (LPL)-Mediated VLDL Catabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipoprotein Isolation
2.3. VLDL Lipolysis Study
2.4. Biochemical Analysis and Agarose Electrophoresis
2.5. Statistical Analysis
3. Results
3.1. The Impact of HDL Subpopulations on VLDL-TG Lipolysis Efficiency
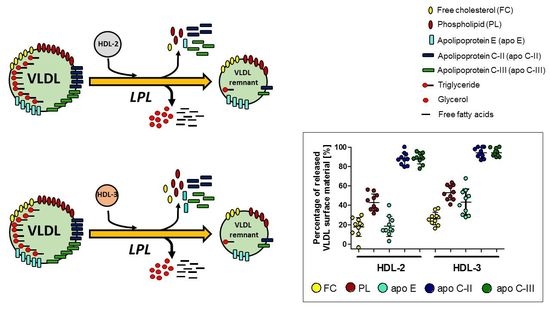
3.2. Impact of HDL Subpopulations on the Release of Surface Lipids from VLDL during Lipolysis
3.3. Impact of HDL Subpopulations on the Release of Exchangeable Apolipoproteins from VLDL during Lipolysis
3.4. Electrophoretic Mobility of VLDL Remnants Produced during Lipolysis in the Absence and Presence of HDL Subpopulations
3.5. Impact of the HDL-2/HDL-3 Cholesterol Ratio in HDLT on the Release of Lipids and Apolipoproteins from VLDL during Lipolysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-Density Lipoprotein (HDL) Functionality and Its Relevance to Atherosclerotic Cardiovascular Disease. Drugs Context 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Woudberg, N.J.; Pedretti, S.; Lecour, S.; Schulz, R.; Vuilleumier, N.; James, R.W.; Frias, M.A. Pharmacological Intervention to Modulate HDL: What Do We Target? Front. Pharmacol. 2018, 8, 989. [Google Scholar] [CrossRef] [Green Version]
- Hernáez, Á.; Soria-Florido, M.T.; Schröder, H.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J. Role of HDL Function and LDL Atherogenicity on Cardiovascular Risk: A Comprehensive Examination. PLoS ONE 2019, 14, e0218533. [Google Scholar] [CrossRef] [PubMed]
- Duparc, T.; Ruidavets, J.B.; Genoux, A.; Ingueneau, C.; Najib, S.; Ferrières, J.; Perret, B.; Martinez, L.O. Serum Level of HDL Particles Are Independently Associated with Long-Term Prognosis in Patients with Coronary Artery Disease: The GENES Study. Sci. Rep. 2020, 10, 8138. [Google Scholar] [CrossRef]
- Martin, S.S.; Jones, S.R.; Toth, P.P. High-Density Lipoprotein Subfractions: Current Views and Clinical Practice Applications. Trends Endocrinol. Metab. 2014, 25, 329–336. [Google Scholar] [CrossRef]
- Chiesa, S.T.; Charakida, M. High-Density Lipoprotein Function and Dysfunction in Health and Disease. Cardiovasc. Drugs Ther. 2019, 33, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontush, A. HDL and Reverse Remnant-Cholesterol Transport (RRT): Relevance to Cardiovascular Disease. Trends Mol. Med. 2020, 12, 1086–1100. [Google Scholar] [CrossRef]
- Chapman, M.; Ginsberg, H.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A. Triglyceride-Rich Lipoproteins and High-Density Lipoprotein Cholesterol in Patients at High Risk of Cardiovascular Disease: Evidence and Guidance for Management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packard, C.J.; Boren, J.; Taskinen, M.R. Causes and Consequences of Hypertriglyceridemia. Front. Endocrinol. (Lausanne) 2020, 11, 252. [Google Scholar] [CrossRef]
- Kei, A.A.; Filippatos, T.D.; Tsimihodimos, V.; Elisaf, M.S. A Review of the Role of Apolipoprotein C-II in Lipoprotein Metabolism and Cardiovascular Disease. Metabolism 2012, 61, 906–921. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.M.M.; Barrett, P.H.R.; Chan, D.C.; Watts, G.F. Apolipoprotein C-III: Understanding an Emerging Cardiovascular Risk Factor. Clin. Sci. 2008, 114, 611–624. [Google Scholar] [CrossRef] [Green Version]
- Dominiczak, M.H.; Caslake, M.J. Apolipoproteins: Metabolic Role and Clinical Biochemistry Applications. Ann. Clin. Biochem. 2011, 48, 498–515. [Google Scholar] [CrossRef]
- Björnson, E.; Adiels, M.; Taskinen, M.-R.; Borén, J. Kinetics of Plasma Triglycerides in Abdominal Obesity. Curr. Opin. Lipidol. 2016, 28, 11–18. [Google Scholar] [CrossRef]
- Nakajima, K.; Tanaka, A. Atherogenic Postprandial Remnant Lipoproteins; VLDL Remnants as a Causal Factor in Atherosclerosis. Clin. Chim. Acta 2018, 478, 200–215. [Google Scholar] [CrossRef]
- Wieczorek, E.; Ćwiklińska, A.; Kuchta, A.; Kortas-Stempak, B.; Gliwińska, A.; Jankowski, M. Decreased Efficiency of Very-Low-Density Lipoprotein Lipolysis Is Linked to Both Hypertriglyceridemia and Hypercholesterolemia, but It Can Be Counteracted by High-Density Lipoprotein. Nutrients 2021, 13, 1224. [Google Scholar] [CrossRef]
- McPherson, P.A.C.; Young, I.S.; McKibben, B.; McEneny, J. High Density Lipoprotein Subfractions: Isolation, Composition, and Their Duplicitous Role in Oxidation. J. Lipid Res. 2007, 48, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ćwiklińska, A.; Cackowska, M.; Wieczorek, E.; Król, E.; Kowalski, R.; Kuchta, A.; Kortas-Stempak, B.; Gliwińska, A.; Dąbkowski, K.; Zielińska, J.; et al. Progression of Chronic Kidney Disease Affects HDL Impact on Lipoprotein Lipase (LPL)-Mediated VLDL Lipolysis Efficiency. Kidney Blood Press. Res. 2018, 43, 970–978. [Google Scholar] [CrossRef]
- Ćwiklińska, A.; Kortas-Stempak, B.; Gliwińska, A.; Pacanis, A.; Kuchta, A.; Wróblewska, M. Interaction Between VLDL and Phosphatidylcholine Liposomes Generates New Gamma-LpE-like Particles. Lipids 2014, 49, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patsch, J.R.; Karlin, J.B.; Scott, L.W.; Smith, L.C.; Gotto, A.M. Inverse Relationship between Blood Levels of High Density Lipoprotein Subfraction 2 and Magnitude of Postprandial Lipemia. Proc. Natl. Acad. Sci. USA 1983, 80, 1449–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, M.; Darabi, M.; Tubeuf, E.; Canicio, A.; Lhomme, M.; Frisdal, E.; Lanfranchi-Lebreton, S.; Matheron, L.; Rached, F.; Ponnaiah, M.; et al. Free Cholesterol Transfer to High-Density Lipoprotein ( HDL ) upon Triglyceride Lipolysis Underlies the U-Shape Relationship between HDL-Cholesterol and Cardiovascular Disease. Eur. J. Prev. Cardiol. 2020, 27, 1606–1616. [Google Scholar] [CrossRef]
- Chung, B.H.; Dashti, N. Lipolytic Remnants of Human VLDL Produced in Vitro: Effect of HDL Levels in the Lipolysis Mixtures on the ApoCs to ApoE Ratio and Metabolic Properties of VLDL Core Remnants. J. Lipid Res. 2000, 41, 285–297. [Google Scholar] [CrossRef]
- Feng, M.; Darabi, M.; Lhomme, M.; Tubeuf, E.; Canicio, A.; Brerault, J.; Medadje, N. Phospholipid Transfer to High-Density Lipoprotein (HDL) upon Triglyceride Lipolysis Is Directly Correlated with HDL-Cholesterol Levels and Is Not Associated with Cardiovascular Risk. Atherosclerosis 2021, 324, 1–8. [Google Scholar] [CrossRef]
- Murdoch, S.J.; Breckenridge, W.C. Influence of Lipoprotein Lipase and Hepatic Lipase on the Transformation of VLDL and HDL during Lipolysis of VLDL. Atherosclerosis 1995, 118, 193–212. [Google Scholar] [CrossRef]
- Taskinena, M.R.; Kashyap, M.L.; Srivastava, L.S.; Ashraf, M.; Johnson, J.D.; Perisutti, G.; Brady, D.; Glueck, C.J.; Jackson, R.L. In Vitro Catabolism of Human Plasma Very Low Density Lipoproteins. Effects of VLDL Concentration on the Interconversion of High Density Lipoprotein Subfractions. Atherosclerosis 1982, 41, 381–394. [Google Scholar] [CrossRef]
- Glangeaud-Freudenthal, M.C.; Burdin, J.; Ayrault-Jarrier, M.; Polonovski, J. Redistribution of Apolipoproteins C Removed from Human Very Low Density Lipoprotein during in Vitro Lipolysis by Lipoprotein Lipase. Biochimie 1981, 63, 485–494. [Google Scholar] [CrossRef]
- Murdoch, S.J.; Breckenridge, W.C. Effect of Lipid Transfer Proteins on Lipoprotein Lipase Induced Transformation of VLDL and HDL. Biochim. Biophys. Acta Lipids Lipid Metab. 1996, 1303, 222–232. [Google Scholar] [CrossRef]
- Eisenberg, S.; Patsch, J.R.; Sparrow, J.T.; Gotto, A.M.; Olivercrona, T. Very Low Density Lipoprotein Removal of Apolipoproteins C-II and C-III-1 During Lipolysis in vitro. J. Biol. Chem. 1979, 254, 12603–12608. [Google Scholar] [CrossRef]
- Yamazaki, A.; Ohkawa, R.; Yamagata, Y.; Horiuchi, Y.; Lai, S.J.; Kameda, T.; Ichimura, N.; Tohda, S.; Tozuka, M. Apolipoprotein C-II and C-III Preferably Transfer to Both HDL2 and the Larger HDL3 from VLDL. Biol. Chem. 2021, 402, 439–449. [Google Scholar] [CrossRef]
- Ćwiklińska, A.; Gliwińska, A.; Senderowska, Z.; Kortas-Stempak, B.; Kuchta, A.; Dąbkowski, K.; Jankowski, M. Impact of Phosphatidylcholine Liposomes on the Compositional Changes of VLDL during Lipoprotein Lipase (LPL)-Mediated Lipolysis. Chem. Phys. Lipids 2016, 195, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, D.M.; Tajmir-Riahi, H.A. Study on the Interaction of Cationic Lipids with Bovine Serum Albumin. J. Phys. Chem. B 2010, 114, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Deckelbaum, R.; Eisenberg, S.; Fainaru, M.; Barenholz, Y.; Olivecrona, T. In Vitro Production of Human Plasma Low Density Lipoprotein-like Particles. J. Biol. Chem. 1979, 254, 6079–6087. [Google Scholar] [CrossRef]
- Tam, S.P.; Dory, L.; Rubinstein, D. Fate of Apolipoproteins C-II, C-III, and E during Lipolysis of Human Very Low Density Lipoproteins in Vitro. J. Lipid Res. 1981, 22, 641–651. [Google Scholar] [CrossRef]
- Camont, L.; Lhomme, M.; Rached, F.; Le Goff, W.; Nègre-Salvayre, A.; Salvayre, R.; Calzada, C.; Lagarde, M.; Chapman, M.J.; Kontush, A. Small, Dense High-Density Lipoprotein-3 Particles Are Enriched in Negatively Charged Phospholipids: Relevance to Cellular Cholesterol Efflux, Antioxidative, Antithrombotic, Anti-Inflammatory, and Antiapoptotic Functionalities. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2715–2723. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Therond, P.; Zerrad, A.; Couturier, M.; Négre-Salvayre, A.; De Souza, J.A.; Chantepie, S.; Chapman, M.J. Preferential Sphingosine-1-Phosphate Enrichment and Sphingomyelin Depletion Are Key Features of Small Dense HDL3 Particles: Relevance to Antiapoptotic and Antioxidative Activities. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1843–1849. [Google Scholar] [CrossRef] [Green Version]
- Arimoto, I.; Saito, H.; Kawashima, Y.; Miyajima, K.; Handa, T. Effects of Sphingomyelin and Cholesterol on Lipoprotein Lipase-Mediated Lipolysis in Lipid Emulsions. J. Lipid Res. 1998, 39, 143–151. [Google Scholar] [CrossRef]
- Morita, S.Y.; Okuhira, K.; Tsuchimoto, N.; Vertut-Doï, A.; Saito, H.; Nakano, M.; Handa, T. Effects of Sphingomyelin on Apolipoprotein E- and Lipoprotein Lipase-Mediated Cell Uptake of Lipid Particles. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1631, 169–176. [Google Scholar] [CrossRef]
- Asztalos, B.F.; Horvath, K.V.; Kajinami, K.; Nartsupha, C.; Cox, C.E.; Batista, M.; Schaefer, E.J.; Inazu, A.; Mabuchi, H. Apolipoprotein Composition of HDL in Cholesteryl Ester Transfer Protein Deficiency. J. Lipid Res. 2004, 45, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Salinas, C.A.A.; Chapman, M.J. Remnant Lipoproteins: Are They Equal to or More Atherogenic than LDL? Curr. Opin. Lipidol. 2020, 31, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Urbina, A.; Rull, A.; Ordóñez-Llanos, J.; Sánchez-Quesada, J.L. Electronegative LDL: An Active Player in Atherogenesis or a By- Product of Atherosclerosis? Curr. Med. Chem. 2019, 26, 1665–1679. [Google Scholar] [CrossRef]
- Chen, W.Y.; Chen, Y.F.; Chan, H.C.; Chung, C.H.; Peng, H.Y.; Ho, Y.C.; Chen, C.H.; Chang, K.C.; Tang, C.H.; Lee, A.S. Role of Apolipoprotein E in Electronegative Low-Density Lipoprotein-Induced Mitochondrial Dysfunction in Cardiomyocytes. Metabolism 2020, 107, 154227. [Google Scholar] [CrossRef]
- Franceschini, G.; Calabresi, L.; Colombo, C.; Favari, E.; Bernini, F.; Sirtori, C.R. Effects of Fenofibrate and Simvastatin on HDL-Related Biomarkers in Low-HDL Patients. Atherosclerosis 2007, 195, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Collins, D.; Freedman, D.S.; Shalaurova, I.; Schaefer, E.J.; McNamara, J.R.; Bloomfield, H.E.; Robins, S.J. Low-Density Lipoprotein and High-Density Lipoprotein Particle Subclasses Predict Coronary Events and Are Favorably Changed by Gemfibrozil Therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006, 113, 1556–1563. [Google Scholar] [CrossRef] [PubMed]





| Parameter | VLDL | HDLT | HDL-2 | HDL-3 |
|---|---|---|---|---|
| Triglycerides [mg/dL] | 74.2 (52.1–110.3) | 12.9 (10.9–14.9) | 7.5 (5.4–10.7) | 4.6 (4.2–4.9) * |
| Total cholesterol [mg/dL] | 20.7 ± 12.4 | 46.8 ± 19.8 | 29.9 ± 15.3 | 16.7 ± 4.7 ** |
| Free cholesterol [mg/dL] | 6.8 ± 3.5 | 11.3 ± 6.0 | 7.2 ± 4.6 | 3.5 ± 1.3 * |
| Phospholipids [mg/dL] | 25.6 ± 13.2 | 121.4 ± 40.7 | 74.4 ± 34.4 | 44.3 ± 9.1 * |
| Free cholesterol/Phospholipids ratio | 0.265 ± 0.027 | 0.089 ± 0.021 | 0.093 ± 0.018 | 0.078 ± 0.013 *** |
| Apolipoprotein AI [mg/dL] | - | 135.3 ± 40.2 | 82.0 ± 31.3 | 54.9 ± 9.8 * |
| Apolipoprotein B [mg/dL] | 7.3 ± 3.2 | - | - | - |
| Apolipoprotein CII [mg/dL] | 1.7 ± 0.8 | 1.7 ± 0.2 | 1.0 ± 0.3 | 0.7 ± 0.1 |
| Apolipoprotein CIII [mg/dL] | 3.6 (2.7–4.5) | 6.4 (4.6–8.3) | 4.0 (2.6–5.9) | 2.2 (1.9–3.2) * |
| Apolipoprotein E [mg/dL] | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.4 ± 0.3 | 0.2 ± 0.1 * |
| Parameter | HDL-2/HDL-3 Ratio <1.56 (n = 5) | HDL-2/HDL-3 Ratio ≥1.56 (n = 5) | p-Value |
|---|---|---|---|
| Free cholesterol [%] | 27.1 ± 5.1 | 12.2 ± 5.2 | 0.002 |
| Phospholipids [%] | 47.7 ± 2.4 | 40.8 ± 6.1 | 0.044 |
| Apolipoprotein E [%] | 36.0 ± 13.9 | 19.5 ± 6.2 | 0.042 |
| Apolipoprotein CII [%] | 95.0 ± 5.0 | 88.2 ± 2.6 | 0.030 |
| Apolipoprotein CIII [%] | 90.6 ± 7.2 | 93.1 ± 4.6 | 0.529 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieczorek, E.; Ćwiklińska, A.; Kuchta, A.; Kortas-Stempak, B.; Gliwińska, A.; Jankowski, M. The Differential Effects of HDL Subpopulations on Lipoprotein Lipase (LPL)-Mediated VLDL Catabolism. Biomedicines 2021, 9, 1839. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9121839
Wieczorek E, Ćwiklińska A, Kuchta A, Kortas-Stempak B, Gliwińska A, Jankowski M. The Differential Effects of HDL Subpopulations on Lipoprotein Lipase (LPL)-Mediated VLDL Catabolism. Biomedicines. 2021; 9(12):1839. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9121839
Chicago/Turabian StyleWieczorek, Ewa, Agnieszka Ćwiklińska, Agnieszka Kuchta, Barbara Kortas-Stempak, Anna Gliwińska, and Maciej Jankowski. 2021. "The Differential Effects of HDL Subpopulations on Lipoprotein Lipase (LPL)-Mediated VLDL Catabolism" Biomedicines 9, no. 12: 1839. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9121839