HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity
Abstract
:1. Introduction
2. HDLs
HDL Function
3. Methods of HDL Measurement in the Laboratory
4. Dysfunctional HDLs: From Quantity to Quality
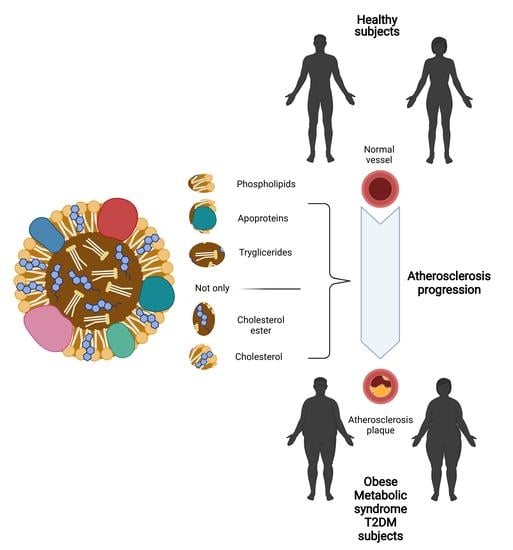
4.1. Obesity
4.2. Diabetes Mellitus Type 2
4.3. Cardiovascular Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuklenyik, Z.; Jones, J.I.; Gardner, M.S.; Schieltz, D.M.; Parks, B.A.; Toth, C.; Rees, J.C.; Andrews, M.L.; Carter, K.; Lehtikoski, A.K.; et al. Core lipid, surface lipid and apolipoprotein composition analysis of lipoprotein particles as a function of particle size in one workflow integrating asymmetric flow field-flow fractionation and liquid chromatography-tandem mass spectrometry. PLoS ONE 2018, 13, e0194797. [Google Scholar] [CrossRef] [Green Version]
- Serban, C.; Muntean, D.M.; Mikhailids, D.P.; Toth, P.P.; Banach, M. Dysfunctional HDL: The journey from savior to slayer. Clin. Lipidol. 2014, 9, 49–59. [Google Scholar] [CrossRef]
- Salazar, J.; Olivar, L.C.; Ramos, E.; Chávez-Castillo, M.; Rojas, J.; Bermúdez, V. Dysfunctional High-Density Lipoprotein: An Innovative Target for Proteomics and Lipidomics. Cholesterol 2015, 2015, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Lindahl, M.; Lhomme, M.; Calabresi, L.; Chapman, M.J.; Davidson, W.S. Structure of HDL: Particle Subclasses and Molecular Components. In Organotypic Models in Drug Development; Springer Science and Business Media LLC: Berlin, Germany, 2014; Volume 224, pp. 3–51. [Google Scholar]
- Chiesa, S.T.; Charakida, M. High-Density Lipoprotein Function and Dysfunction in Health and Disease. Cardiovasc. Drugs Ther. 2019, 33, 207–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothblat, G.H.; Phillips, M.C. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 2010, 21, 229–238. [Google Scholar] [CrossRef]
- Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proia, R.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef]
- Levkau, B. HDL-S1P: Cardiovascular functions, disease-associated alterations, and therapeutic applications. Front. Pharmacol. 2015, 6, 243. [Google Scholar] [CrossRef] [Green Version]
- Kurano, M.; Yatomi, Y. Sphingosine 1-Phosphate and Atherosclerosis. J. Atheroscler. Thromb. 2018, 25, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argraves, K.M.; Argraves, W.S. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid Res. 2007, 48, 2325–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkard, I.; von Eckardstein, A.; Waeber, G.; Vollenweider, P.; Rentsch, K.M. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis 2007, 194, 71–78. [Google Scholar] [CrossRef]
- Tikkanen, M.J.; Vihma, V.; Jauhiainen, M.; Höckerstedt, A.; Helisten, H.; Kaamanen, M. Lipoprotein-associated estrogens. Cardiovasc. Res. 2002, 56, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Herrera, E.; Barbas, C. Vitamin E: action, metabolism and perspectives. J. Physiol. Biochem. 2001, 57, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Cervato, G.; Cestaro, B. Variability in alpha-tocopherol antioxidant activity in the core and surface layers of low- and high-density lipoproteins. J. Nutr. Sci. Vitaminol. 1999, 45, 39–48. [Google Scholar] [CrossRef]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Burri, B.J. Beta-cryptoxanthin as a source of vitamin A. J. Sci. Food Agric. 2014, 95, 1786–1794. [Google Scholar] [CrossRef]
- Waldie, S.; Sebastiani, F.; Browning, K.; Maric, S.; Lind, T.K.; Yepuri, N.; Darwish, T.A.; Moulin, M.; Strohmeier, G.; Pichler, H.; et al. Lipoprotein ability to exchange and remove lipids from model membranes as a function of fatty acid saturation and presence of cholesterol. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158769. [Google Scholar] [CrossRef]
- Cazzola, R.; Cassani, E.; Barichella, M.; Cestaro, B. Impaired fluidity and oxidizability of HDL hydrophobic core and amphipathic surface in dyslipidemic men. Metabolism 2013, 62, 986–991. [Google Scholar] [CrossRef]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, D.L.; Davidson, W.S.; Lund-Katz, S.; Phillips, M.C. Effects of the Neutral Lipid Content of High Density Lipoprotein on Apolipoprotein A-I Structure and Particle Stability. J. Biol. Chem. 1995, 270, 26910–26917. [Google Scholar] [CrossRef] [Green Version]
- Curtiss, L.K.; Bonnet, D.J.; Rye, K.-A. The conformation of apolipoprotein A-I in high-density lipoproteins is influenced by core lipid composition and particle size: A surface plasmon resonance study. Biochemistry 2000, 39, 5712–5721. [Google Scholar] [CrossRef]
- Bolin, D.J.; Jonas, A. Sphingomyelin Inhibits the Lecithin-Cholesterol Acyltransferase Reaction with Reconstituted High Density Lipoproteins by Decreasing Enzyme Binding. J. Biol. Chem. 1996, 271, 19152–19158. [Google Scholar] [CrossRef] [Green Version]
- Kontush, A.; Chapman, M.J. Functionally Defective High-Density Lipoprotein: A New Therapeutic Target at the Crossroads of Dyslipidemia, Inflammation, and Atherosclerosis. Pharmacol. Rev. 2006, 58, 342–374. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Lüscher, T.F.; Landmesser, U. Molecular mechanisms of vascular effects of High-density lipoprotein: Alterations in cardiovascular disease. EMBO Mol. Med. 2012, 4, 251–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Barter, P.J.; Nicholls, S.; Rye, K.-A.; Anantharamaiah, G.M.; Navab, M.; Fogelman, A.M. Antiinflammatory Properties of HDL. Circ. Res. 2004, 95, 764–772. [Google Scholar] [CrossRef]
- Karathanasis, S.K.; Freeman, L.A.; Gordon, S.M.; Remaley, A.T. The Changing Face of HDL and the Best Way to Measure It. Clin. Chem. 2017, 63, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Bardagjy, A.S.; Steinberg, F.M. Relationship between HDL Functional Characteristics and Cardiovascular Health and Potential Impact of Dietary Patterns: A Narrative Review. Nutrients 2019, 11, 1231. [Google Scholar] [CrossRef] [Green Version]
- Marques, L.R.; Diniz, T.A.; Antunes, B.M.; Rossi, F.E.; Caperuto, E.; Lira, F.S.; Goncalves, D. Reverse Cholesterol Transport: Molecular Mechanisms and the Non-medical Approach to Enhance HDL Cholesterol. Front. Physiol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Phillips, M.C. Molecular Mechanisms of Cellular Cholesterol Efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.G.; de la Llera-Moya, M.; Swarnakar, S.; Monzo, P.; Klein, S.M.; Connelly, M.A.; Johnson, W.J.; Williams, D.L.; Rothblat, G.H. High Density Lipoprotein Phospholipid Composition Is a Major Determinant of the Bi-directional Flux and Net Movement of Cellular Free Cholesterol Mediated by Scavenger Receptor BI. J. Biol. Chem. 2000, 275, 36596–36604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litman, B.J.; Lewis, E.N.; Levin, I.W. Packing characteristics of highly unsaturated bilayer lipids: Raman spectroscopic studies of multilamellar phosphatidylcholine dispersions. Biochemistry 1991, 30, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Davidson, W.S.; Gillotte, K.L.; Lund-Katz, S.; Johnson, W.J.; Rothblat, G.H.; Phillips, M.C. The Effect of High Density Lipoprotein Phospholipid Acyl Chain Composition on the Efflux of Cellular Free Cholesterol. J. Biol. Chem. 1995, 270, 5882–5890. [Google Scholar] [CrossRef] [Green Version]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef] [Green Version]
- Sugano, M.; Tsuchida, K.; Makino, N. High-Density Lipoproteins Protect Endothelial Cells from Tumor Necrosis Factor-α-Induced Apoptosis. Biochem. Biophys. Res. Commun. 2000, 272, 872–876. [Google Scholar] [CrossRef]
- Bowry, V.; Stanley, K.K.; Stocker, R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. USA 1992, 89, 10316–10320. [Google Scholar] [CrossRef] [Green Version]
- Proudfoot, J.M.; Barden, A.E.; Loke, W.M.; Croft, K.D.; Puddey, I.B.; Mori, T.A. HDL is the major lipoprotein carrier of plasma F2-isoprostanes. J. Lipid Res. 2009, 50, 716–722. [Google Scholar] [CrossRef] [Green Version]
- Christison, J.; Karjalainen, A.; Brauman, J.; Bygrave, F.; Stocker, R. Rapid reduction and removal of HDL- but not LDL-associated cholesteryl ester hydroperoxides by rat liver perfused in situ. Biochem. J. 1996, 314, 739–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerrad-Saadi, A.; Therond, P.; Chantepie, S.; Couturier, M.; Rye, K.-A.; Chapman, M.J.; Kontush, A. HDL3-Mediated Inactivation of LDL-Associated Phospholipid Hydroperoxides Is Determined by the Redox Status of Apolipoprotein A-I and HDL Particle Surface Lipid Rigidity. Arter. Thromb. Vasc. Biol. 2009, 29, 2169–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Zhou, W.; Wang, B.; Zhu, H.; Ye, L.; Feng, M. Antioxidant effect of apolipoprotein A-I on high-fat diet-induced non-alcoholic fatty liver disease in rabbits. Acta Biochim. Biophys. Sin. 2013, 45, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Soran, H.; Schofield, J.D.; Durrington, P.N. Antioxidant properties of HDL. Front. Pharmacol. 2015, 6, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenson, R.S.; Brewer, H.B., Jr.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef]
- März, W.; Kleber, M.E.; Scharnagl, H.; Speer, T.; Zewinger, S.; Ritsch, A.; Parhofer, K.G.; Von Eckardstein, A.; Landmesser, U.; Laufs, U. HDL cholesterol: Reappraisal of its clinical relevance. Clin. Res. Cardiol. 2017, 106, 663–675. [Google Scholar] [CrossRef] [Green Version]
- Hafiane, A.; Genest, J. High density lipoproteins: Measurement techniques and potential biomarkers of cardiovascular risk. BBA Clin. 2015, 3, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.G.; Myers, G.L.; Sakurabayashi, I.; Bachmann, L.M.; Caudill, S.P.; Dziekonski, A.; Edwards, S.; Kimberly, M.M.; Korzun, W.J.; Leary, E.T.; et al. Seven Direct Methods for Measuring HDL and LDL Cholesterol Compared with Ultracentrifugation Reference Measurement Procedures. Clin. Chem. 2010, 56, 977–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitla, A.; Naresh, S.; Sachan, A. High-density lipoprotein: Quality versus quantity in type 2 diabetes mellitus. J. Clin. Sci. Res. 2019, 8, 193. [Google Scholar] [CrossRef]
- Ito, F.; Ito, T. High-Density Lipoprotein (HDL) Triglyceride and Oxidized HDL: New Lipid Biomarkers of Lipoprotein-Related Atherosclerotic Cardiovascular Disease. Antioxidants 2020, 9, 362. [Google Scholar] [CrossRef]
- Takeda, H.; Koike, T.; Izumi, Y.; Yamada, T.; Yoshida, M.; Shiomi, M.; Fukusaki, E.; Bamba, T. Lipidomic analysis of plasma lipoprotein fractions in myocardial infarction-prone rabbits. J. Biosci. Bioeng. 2015, 120, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Furtado, J.D.; Yamamoto, R.; Melchior, J.T.; Andraski, A.B.; Gamez-Guerrero, M.; Mulcahy, P.; He, Z.; Cai, T.; Davidson, W.S.; Sacks, F.M. Distinct Proteomic Signatures in 16 HDL (High-Density Lipoprotein) Subspecies. Arter. Thromb. Vasc. Biol. 2018, 38, 2827–2842. [Google Scholar] [CrossRef] [Green Version]
- Von Zychlinski, A.; Kleffmann, T. Dissecting the proteome of lipoproteins: New biomarkers for cardiovascular diseases? Transl. Proteom. 2015, 7, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, A.; Piuri, G.; Della Porta, M.; Mazzucchelli, S.; Bonizzi, A.; Truffi, M.; Sevieri, M.; Allevi, R.; Corsi, F.; Cazzola, R.; et al. Raman spectroscopy characterization of the major classes of plasma lipoproteins. Vib. Spectrosc. 2020, 109, 103073. [Google Scholar] [CrossRef]
- Clamp, A.G.; Ladha, S.; Clark, D.C.; Grimble, R.F. Determination of membrane fluidity: A comparison of biophysical methods. Biochem. Soc. Trans. 1994, 22, 369S. [Google Scholar] [CrossRef] [Green Version]
- Cazzola, R.; Piuri, G.; Garziano, M.; Cassani, E.; Boggio, A.; Barichella, M.; Muzio, F.; Cestaro, B. The Role of Triacylglycerol in the Oxidizability of Low Density Lipoproteins and High Density Lipoproteins: Possible Contributions to Atherosclerosis in Metabolic Syndrome. J. Obes. Weight. Loss Ther. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. High-Density Lipoprotein Subfractions—What the Clinicians Need to Know. Cardiology 2013, 124, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Catapano, A.L. Treating High Density Lipoprotein Cholesterol (HDL-C): Quantity versus Quality. Curr. Pharm. Des. 2013, 19, 3841–3857. [Google Scholar] [CrossRef]
- Femlak, M.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Rysz, J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis. 2017, 16, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nessler, K.; Windak, A.; Grzybczak, R.; Nessler, M.; Siniarski, A.; Gajos, G. High-density lipoprotein (HDL) cholesterol—More complicated than we think? Ann. Agric. Environ. Med. 2018, 25, 517–526. [Google Scholar] [CrossRef]
- Srivastava, R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol. Cell. Biochem. 2017, 440, 167–187. [Google Scholar] [CrossRef]
- Farbstein, D.; Levy, A.P. HDL dysfunction in diabetes: Causes and possible treatments. Expert Rev. Cardiovasc. Ther. 2012, 10, 353–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Novel Insights into the Pathogenesis and Management of the Metabolic Syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Ahn, N.; Kim, K. High-density lipoprotein cholesterol (HDL-C) in cardiovascular disease: Effect of exercise training. Integr. Med. Res. 2016, 5, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Peng, D.-Q. New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 2011, 10, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Watts, G.F.; Johnson, A.G.; Chan, D.C.; Ooi, E.M.M.; Rye, K.-A.; Serone, A.P.; Barrett, P.H.R. High-Density Lipoprotein (HDL) Transport in the Metabolic Syndrome: Application of a New Model for HDL Particle Kinetics. J. Clin. Endocrinol. Metab. 2006, 91, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Denimal, D.; Monier, S.; Brindisi, M.-C.; Petit, J.-M.; Bouillet, B.; Nguyen, A.; Demizieux, L.; Simoneau, I.; De Barros, J.-P.P.; Vergès, B.; et al. Impairment of the Ability of HDL from Patients with Metabolic Syndrome but Without Diabetes Mellitus to Activate eNOS. Arter. Thromb. Vasc. Biol. 2017, 37, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Hui, N.; Barter, P.J.; Ong, K.-L.; Rye, K.-A. Altered HDL metabolism in metabolic disorders: Insights into the therapeutic potential of HDL. Clin. Sci. 2019, 133, 2221–2235. [Google Scholar] [CrossRef]
- Lamarche, B.; Rashid, S.; Lewis, G.F. HDL metabolism in hypertriglyceridemic states: An overview. Clin. Chim. Acta 1999, 286, 145–161. [Google Scholar] [CrossRef]
- Rashid, S.; Uffelman, K.D.; Lewis, G.F. The mechanism of HDL lowering in hypertriglyceridemic, insulin-resistant states. J. Diabetes Its Complicat. 2002, 16, 24–28. [Google Scholar] [CrossRef]
- Lucero, D.; Sviridov, D.; Freeman, L.; López, G.I.; Fassio, E.; Remaley, A.T.; Schreier, L. Increased cholesterol efflux capacity in metabolic syndrome: Relation with qualitative alterations in HDL and LCAT. Atherosclerosis 2015, 242, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.; Frisdal, E.; Bittar, R.; Le Goff, W.; Bruckert, E.; Lesnik, P.; Guerin, M.; Giral, P. Association of Cholesterol Efflux Capacity with Clinical Features of Metabolic Syndrome: Relevance to Atherosclerosis. J. Am. Hear. Assoc. 2016, 5, 004808. [Google Scholar] [CrossRef] [PubMed]
- Kota, S.K.; Kota, S.K.; Krishna, S.V.S.; Meher, L.K.; Modi, K.D.; Jammula, S. Implications of serum paraoxonase activity in obesity, diabetes mellitus, and dyslipidemia. Indian J. Endocrinol. Metab. 2013, 17, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Montefusco, L.; D’Addio, F.; Loretelli, C.; Ben Nasr, M.; Garziano, M.; Rossi, A.; Pastore, I.; Plebani, L.; Lunati, M.E.; Bolla, A.M.; et al. Anti-inflammatory effects of diet and caloric restriction in metabolic syndrome. J. Endocrinol. Investig. 2021, 1–9. [Google Scholar] [CrossRef]
- Yoon, J.-H. Prevalence of Obesity and Incidence of Obesity-Related Comorbidities in Koreans Based on National Health Insurance Service Health Checkup Data 2006–2015 (J Obes Metab Syndr 2018;27:46–52). J. Obes. Metab. Syndr. 2018, 27, 195–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genua, I.; Ramos, A.; Caimari, F.; Balagué, C.; Sánchez-Quesada, J.L.; Pérez, A.; Miñambres, I. Effects of Bariatric Surgery on HDL Cholesterol. Obes. Surg. 2020, 30, 1793–1798. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tardif, I.; Auclair, A.; Poirier, P. Effects of bariatric surgery on lipid-lipoprotein profile. Metabolism 2021, 115, 154441. [Google Scholar] [CrossRef]
- Chen, C.; Ye, Y.; Zhang, Y.; Pan, X.-F.; Pan, A. Weight change across adulthood in relation to all cause and cause specific mortality: Prospective cohort study. BMJ 2019, 367, l5584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangalore, S.; Fayyad, R.; Laskey, R.; Demicco, D.A.; Messerli, F.H.; Waters, D.D. Body-Weight Fluctuations and Outcomes in Coronary Disease. N. Engl. J. Med. 2017, 376, 1332–1340. [Google Scholar] [CrossRef]
- Gregg, E.W. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Han, K.; Koh, E.S.; Kim, E.S.; Lee, M.-K.; Nam, G.E.; Kwon, H.-S. Weight change and mortality and cardiovascular outcomes in patients with new-onset diabetes mellitus: A nationwide cohort study. Cardiovasc. Diabetol. 2019, 18, 36. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.-Y.; Yan, S.-T.; Wang, M.-L.; Li, Z.-B.; Fang, L.-Q.; Zeng, Q. Associations of body weight and weight change with cardiovascular events and mortality in patients with coronary heart disease. Atherosclerosis 2018, 274, 104–111. [Google Scholar] [CrossRef]
- Navab, M.; Hama, S.Y.; Anantharamaiah, G.; Hassan, K.; Hough, G.P.; Watson, A.D.; Reddy, S.T.; Sevanian, A.; Fonarow, G.; Fogelman, A.M. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J. Lipid Res. 2000, 41, 1495–1508. [Google Scholar] [CrossRef]
- Navab, M.; Hama, S.Y.; Cooke, C.J.; Anantharamaiah, G.; Chaddha, M.; Jin, L.; Subbanagounder, G.; Faull, K.F.; Reddy, S.T.; Miller, N.E.; et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Step 1. J. Lipid Res. 2000, 41, 1481–1494. [Google Scholar] [CrossRef]
- Wong, N.K.P.; Nicholls, S.J.; Tan, J.T.M.; Bursill, C.A. The Role of High-Density Lipoproteins in Diabetes and Its Vascular Complications. Int. J. Mol. Sci. 2018, 19, 1680. [Google Scholar] [CrossRef] [Green Version]
- Ebtehaj, S.; Gruppen, E.G.; Parvizi, M.; Tietge, U.J.F.; Dullaart, R.P.F. The anti-inflammatory function of HDL is impaired in type 2 diabetes: Role of hyperglycemia, paraoxonase-1 and low grade inflammation. Cardiovasc. Diabetol. 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asleh, R.; Blum, S.; Kalet-Litman, S.; Alshiek, J.; Miller-Lotan, R.; Asaf, R.; Rock, W.; Aviram, M.; Milman, U.; Shapira, C.; et al. Correction of HDL Dysfunction in Individuals With Diabetes and the Haptoglobin 2-2 Genotype. Diabetes 2008, 57, 2794–2800. [Google Scholar] [CrossRef] [Green Version]
- Aroner, S.A.; Furtado, J.D.; Sacks, F.M.; Tsai, M.Y.; Mukamal, K.J.; McClelland, R.L.; Jensen, M.K. Apolipoprotein C-III and its defined lipoprotein subspecies in relation to incident diabetes: The Multi-Ethnic Study of Atherosclerosis. Diabetologia 2019, 62, 981–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-Q.; Liu, X.-C.; Lo, K.; Liu, L.; Yu, Y.-L.; Chen, C.-L.; Huang, J.-Y.; Feng, Y.-Q.; Zhang, B. The U Shaped Relationship between High-Density Lipoprotein Cholesterol and All-Cause or Cause-Specific Mortality in Adult Population. Clin. Interv. Aging 2020, 15, 1883–1896. [Google Scholar] [CrossRef]
- Yi, S.-W.; Park, S.-J.; Yi, J.-J.; Ohrr, H.; Kim, H. High-density lipoprotein cholesterol and all-cause mortality by sex and age: A prospective cohort study among 15.8 million adults. Int. J. Epidemiol. 2020, dyaa243. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Annema, W.; von Eckardstein, A. Dysfunctional high-density lipoproteins in coronary heart disease: Implications for diagnostics and therapy. Transl. Res. 2016, 173, 30–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef]
- Quispe, R.; Elshazly, M.B.; Zhao, D.; Toth, P.P.; Puri, R.; Virani, S.S.; Blumenthal, R.S.; Martin, S.S.; Jones, S.R.; Michos, E.D. Total cholesterol/HDL-cholesterol ratio discordance with LDL-cholesterol and non-HDL-cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: The ARIC study. Eur. J. Prev. Cardiol. 2020, 27, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Rana, J.S.; Stroes, E.S.; Després, J.-P.; Shah, P.K.; Kastelein, J.J.; Wareham, N.J.; Boekholdt, S.M.; Khaw, K.-T. Beyond Low-Density Lipoprotein Cholesterol. J. Am. Coll. Cardiol. 2009, 55, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, K.K.; Cannon, C.P.; Cairns, R.; Morrow, D.A.; Ridker, P.M.; Braunwald, E. Prognostic Utility of ApoB/AI, Total Cholesterol/HDL, Non-HDL Cholesterol, or hs-CRP as Predictors of Clinical Risk in Patients Receiving Statin Therapy After Acute Coronary Syndromes. Arter. Thromb. Vasc. Biol. 2009, 29, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Rader, D.J.; Alexander, E.T.; Weibel, G.L.; Billheimer, J.; Rothblat, G.H. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009, 50, S189–S194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; De Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Annema, W.; Dikkers, A.; De Boer, J.F.; Van Greevenbroek, M.M.J.; Van Der Kallen, C.J.H.; Schalkwijk, C.G.; Stehouwer, C.D.A.; Dullaart, R.P.F.; Tietge, U.J.F. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: The CODAM study. Sci. Rep. 2016, 6, 27367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events. Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Shea, S.; Stein, J.H.; Jorgensen, N.W.; McClelland, R.L.; Tascau, L.; Shrager, S.; Heinecke, J.W.; Yvan-Charvet, L.; Tall, A.R. Cholesterol Mass Efflux Capacity, Incident Cardiovascular Disease, and Progression of Carotid Plaque. Arter. Thromb. Vasc. Biol. 2019, 39, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-M.; Tang, W.H.W.; Mosior, M.K.; Huang, Y.; Wu, Y.; Matter, W.; Gao, V.; Schmitt, D.; DiDonato, J.A.; Fisher, E.; et al. Paradoxical Association of Enhanced Cholesterol Efflux With Increased Incident Cardiovascular Risks. Arter. Thromb. Vasc. Biol. 2013, 33, 1696–1705. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, L.; Bonci, E.; Andreoli, G.; Romaggioli, S.; Di Miscio, R.; Lombardo, C.; Chiesa, C. Association of serum triglyceride-to-HDL cholesterol ratio with carotid artery intima-media thickness, insulin resistance and nonalcoholic fatty liver disease in children and adolescents. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.I.; Streja, E.; Soohoo, M.; Kim, T.W.; Rhee, C.M.; Kovesdy, C.P.; Kashyap, M.L.; Vaziri, N.D.; Kalantar-Zadeh, K.; Moradi, H. Association of Serum Triglyceride to HDL Cholesterol Ratio with All-Cause and Cardiovascular Mortality in Incident Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2017, 12, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.-K.; Lee, H.S.; Lee, Y.-J. Triglyceride to HDL-cholesterol ratio and the incidence risk of type 2 diabetes in community dwelling adults: A longitudinal 12-year analysis of the Korean Genome and Epidemiology Study. Diabetes Res. Clin. Pr. 2020, 163, 108150. [Google Scholar] [CrossRef] [PubMed]
- Yunke, Z.; Guoping, L.; Zhenyue, C. Triglyceride-to-HDL cholesterol ratio. Herz 2014, 39, 105–110. [Google Scholar] [CrossRef]
- Lee, Y.; Park, S.; Lee, S.; Kim, Y.; Kang, M.W.; Cho, S.; Park, S.; Han, K.; Kim, Y.C.; Han, S.S.; et al. Lipid profiles and risk of major adverse cardiovascular events in CKD and diabetes: A nationwide population-based study. PLoS ONE 2020, 15, e0231328. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.-Y.; Zheng, Y.-Y.; Tang, J.-N.; Yang, X.-M.; Guo, Q.-Q.; Zhang, J.-C.; Cheng, M.-D.; Song, F.-H.; Liu, Z.-Y.; Wang, K.; et al. Triglyceride to high-density lipoprotein cholesterol ratio as a predictor of long-term mortality in patients with coronary artery disease after undergoing percutaneous coronary intervention: A retrospective cohort study. Lipids Health Dis. 2019, 18, 210. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.-T.; Zheng, Y.-Y.; Chen, Y.; Yu, Z.-X.; Ma, Y.-T.; Xie, X. Monocyte to high-density lipoprotein cholesterol ratio as long-term prognostic marker in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis. 2019, 18, 180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.-P.; Baituola, G.; Wu, T.-T.; Chen, Y.; Hou, X.-G.; Yang, Y.; Pan, Y.; Ma, X.; Zheng, Y.-Y. An elevated monocyte-to-high-density lipoprotein–cholesterol ratio is associated with mortality in patients with coronary artery disease who have undergone PCI. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Gotto, A.M.; Ruscica, M.; Atkin, S.L.; Butler, A.E.; Banach, M.; Sahebkar, A. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J. Cell. Physiol. 2018, 233, 9237–9246. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonizzi, A.; Piuri, G.; Corsi, F.; Cazzola, R.; Mazzucchelli, S. HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity. Biomedicines 2021, 9, 729. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9070729
Bonizzi A, Piuri G, Corsi F, Cazzola R, Mazzucchelli S. HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity. Biomedicines. 2021; 9(7):729. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9070729
Chicago/Turabian StyleBonizzi, Arianna, Gabriele Piuri, Fabio Corsi, Roberta Cazzola, and Serena Mazzucchelli. 2021. "HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity" Biomedicines 9, no. 7: 729. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9070729