Effect of Intermediate Airway Management on Ventilation Parameters in Simulated Pediatric Out-of-Hospital Cardiac Arrest: Protocol for a Multicenter, Randomized, Crossover Trial
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
2. Materials and Methods
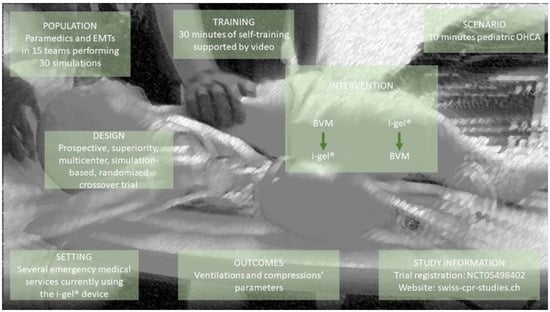
2.1. Study Design and Setting
2.2. Participants, and Inclusion and Exclusion Criteria
2.3. Intra-Cluster Randomization
2.4. Self-Managed Training
2.5. Study Groups, Second Randomization and Concealment of Allocation
2.6. Manikin and Resuscitation Equipment
2.7. Pediatric Cardiac Arrest Scenario
2.8. Outcomes
- -
- The proportion and the number of ventilations below (<45 mL), within (45–72 mL) and over (>72 mL) the target volume;
- -
- The time to the first efficient ventilation (≥45 mL);
- -
- The time to the first compression;
- -
- The CCF;
- -
- The chest compression rate;
- -
- The proportion of chest compressions below (<100/min), within (100–120/min) and over (>120/min) the target rate [54];
- -
- The chest compression depth;
- -
- The proportion of chest compressions below (<4.3 cm) and within (≥4.3 cm) the target depth [54]; this threshold corresponds to one-third of the manikin’s measured anteroposterior chest depth;
- -
- The proportion of chest compressions below (<3 cm) and within (>3 cm) the manufacturer’s target;
- -
- The proportion of complete chest recoil;
- -
- The time to the first epinephrine injection;
- -
- The proportion of scenarios in which epinephrine is administered within 5 min [9].
2.9. Sample Size Calculation
2.10. Blinding, Bias Minimization, and Data Collection and Extraction
2.11. Statistical Analysis
2.12. Confidentiality
2.13. Criteria for Discontinuing or Modifying Interventions
3. Results
3.1. Milestones
3.2. Protocol Version
3.3. Trial Registration
3.4. Data Curation and Availability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Tham, L.P.; Wah, W.; Phillips, R.; Shahidah, N.; Ng, Y.Y.; Shin, S.D.; Nishiuchi, T.; Wong, K.D.; Ko, P.C.-I.; Khunklai, N.; et al. Epidemiology and Outcome of Paediatric Out-of-Hospital Cardiac Arrests: A Paediatric Sub-Study of the Pan-Asian Resuscitation Outcomes Study (PAROS). Resuscitation 2018, 125, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yu, J.; Chang, H.; Heo, S.; Lee, S.U.; Hwang, S.Y.; Yoon, H.; Cha, W.C.; Shin, T.G.; Kim, T. National Surveillance of Pediatric Out-of-Hospital Cardiac Arrest in Korea: The 10-Year Trend From 2009 to 2018. J. Korean Med. Sci. 2022, 37, e317. [Google Scholar] [CrossRef] [PubMed]
- Holgersen, M.G.; Jensen, T.W.; Breindahl, N.; Kjerulff, J.L.B.; Breindahl, S.H.; Blomberg, S.N.F.; Wolthers, S.A.; Andersen, L.B.; Torp-Pedersen, C.; Mikkelsen, S.; et al. Pediatric Out-of-Hospital Cardiac Arrest in Denmark. Scand. J. Trauma Resusc. Emerg. Med. 2022, 30, 58. [Google Scholar] [CrossRef]
- Lee, J.; Yang, W.-C.; Lee, E.-P.; Huang, J.-L.; Hsiao, H.-J.; Lin, M.-J.; Wu, H.-P. Clinical Survey and Predictors of Outcomes of Pediatric Out-of-Hospital Cardiac Arrest Admitted to the Emergency Department. Sci. Rep. 2019, 9, 7032. [Google Scholar] [CrossRef] [Green Version]
- Ohashi-Fukuda, N.; Fukuda, T.; Doi, K. Association between Time to Advanced Airway Management and Survival during Pediatric Out-of-Hospital Cardiac Arrest. Resusc. Plus 2022, 11, 100260. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Ohshimo, S.; Nation, K.; Van de Voorde, P.; Nuthall, G.; Maconochie, I.; Torabi, N.; Morrison, L.J. International Liaison Committee on Resuscitation (ILCOR) Pediatric Life Support Task Force Advanced Airway Interventions for Paediatric Cardiac Arrest: A Systematic Review and Meta-Analysis. Resuscitation 2019, 138, 114–128. [Google Scholar] [CrossRef]
- Weihing, V.K.; Crowe, E.H.; Wang, H.E.; Ugalde, I.T. Prehospital Airway Management in the Pediatric Patient: A Systematic Review. Acad. Emerg. Med. 2021, 29, 765–771. [Google Scholar] [CrossRef]
- Topjian, A.A.; Raymond, T.T.; Atkins, D.; Chan, M.; Duff, J.P.; Joyner, B.L.; Lasa, J.J.; Lavonas, E.J.; Levy, A.; Mahgoub, M.; et al. Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S469–S523. [Google Scholar] [CrossRef]
- Daigle, C.H.; Fiadjoe, J.E.; Laverriere, E.K.; Bruins, B.B.; Lockman, J.L.; Shults, J.; Krawiec, C.; Harwayne-Gidansky, I.; Page-Goertz, C.; Furlong-Dillard, J.; et al. Difficult Bag-Mask Ventilation in Critically Ill Children Is Independently Associated With Adverse Events. Crit. Care Med. 2020, 48, e744. [Google Scholar] [CrossRef]
- Santos-Folgar, M.; Lafuente-Filgueira, P.; Otero-Agra, M.; Fernández-Méndez, F.; Barcala-Furelos, R.; Trastoy-Quintela, J.; Aranda-García, S.; Fernández-Méndez, M.; Rodríguez-Núñez, A. Quality of Ventilations during Infant Resuscitation: A Simulation Study Comparing Endotracheal Tube with Face Mask. Children 2022, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.J.; Langhan, M.L. Can Providers Use Clinical Skills to Assess the Adequacy of Ventilation in Children During Bag-Valve Mask Ventilation? Pediatr. Emerg. Care 2020, 36, e695–e699. [Google Scholar] [CrossRef] [PubMed]
- Fitz-Clarke, J.R. Fast or Slow Rescue Ventilations: A Predictive Model of Gastric Inflation. Respir. Care 2018, 63, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, V.; Idris, A.H.; Banner, M.J.; Kubilis, P.S.; Band, R.; Williams, J.L.; Lindner, K.H. Respiratory System Compliance Decreases after Cardiopulmonary Resuscitation and Stomach Inflation: Impact of Large and Small Tidal Volumes on Calculated Peak Airway Pressure1Presented, in Part, at the 71st Scientific Sessions of the American Heart Association, Dallas, TX, November, 1998.1. Resuscitation 1998, 38, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ruben, H.; Knudsen, E.J.; Carugati, G. Gastric Inflation in Relation to Airway Pressure. Acta Anaesthesiol. Scand. 1961, 5, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Paal, P.; Neurauter, A.; Loedl, M.; Brandner, J.; Herff, H.; Knotzer, H.; Mitterlechner, T.; von Goedecke, A.; Bale, R.; Lindner, K.H.; et al. Effects of Stomach Inflation on Haemodynamic and Pulmonary Function during Spontaneous Circulation in Pigs. Resuscitation 2009, 80, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Aramendi, E.; Irusta, U. To Interrupt, or Not to Interrupt Chest Compressions for Ventilation: That Is the Question! J. Thorac. Dis. 2016, 8, E121–E123. [Google Scholar] [CrossRef] [PubMed]
- Buis, M.L.; Maissan, I.M.; Hoeks, S.E.; Klimek, M.; Stolker, R.J. Defining the Learning Curve for Endotracheal Intubation Using Direct Laryngoscopy: A Systematic Review. Resuscitation 2016, 99, 63–71. [Google Scholar] [CrossRef]
- Galinski, M.; Wrobel, M.; Boyer, R.; Reuter, P.G.; Ruscev, M.; Debaty, G.; Bagou, G.; Dehours, E.; Bosc, J.; Lorendeau, J.-P.; et al. Risk Factors for Failed First Intubation Attempt in an Out-of-Hospital Setting: A Multicenter Prospective Study. Intern. Emerg. Med. 2022. [Google Scholar] [CrossRef]
- Walas, W.; Aleksandrowicz, D.; Borszewska-Kornacka, M.; Gaszyński, T.; Helwich, E.; Migdał, M.; Piotrowski, A.; Siejka, G.; Szczapa, T.; Bartkowska-Śniatkowska, A. Unanticipated Difficult Airway Management in Children—The Consensus Statement of the Paediatric Anaesthesiology and Intensive Care Section and the Airway Management Section of the Polish Society of Anaesthesiology and Intensive Therapy and the Polish So. Anaesthesiol. Intensive Ther. 2017, 49, 336–349. [Google Scholar] [CrossRef]
- Hansen, M.; Lambert, W.; Guise, J.-M.; Warden, C.R.; Mann, N.C.; Wang, H. Out-of-Hospital Pediatric Airway Management in the United States. Resuscitation 2015, 90, 104–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, G.S.; Patanwala, A.E.; Leetch, A.N.; Mendelson, J.S.; Hurst, N.B.; Sakles, J.C. Intubation During Pediatric Cardiac Arrest in the Emergency Department Is Associated With Reduced First-Pass Success. Pediatr. Emerg. Care 2022, 38, e1271–e1276. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, H.; Kunitani, Y.; Goto, T.; Okamoto, H.; Hagiwara, Y.; Watase, H.; Hasegawa, K. Japanese Emergency Medicine Network Investigators Association Between Repeated Tracheal Intubation Attempts and Adverse Events in Children in the Emergency Department. Pediatr. Emerg. Care 2022, 38, e563–e568. [Google Scholar] [CrossRef] [PubMed]
- Suppan, L.; Fehlmann, C.A.; Stuby, L.; Suppan, M. The Importance of Acknowledging an Intermediate Category of Airway Management Devices in the Prehospital Setting. Healthcare 2022, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Leventis, C.; Chalkias, A.; Sampanis, M.A.; Foulidou, X.; Xanthos, T. Emergency Airway Management by Paramedics: Comparison between Standard Endotracheal Intubation, Laryngeal Mask Airway, and I-Gel. Eur. J. Emerg. Med. 2014, 21, 371–373. [Google Scholar] [CrossRef]
- Beylacq, L.; Bordes, M.; Semjen, F.; Cros, A.-M. The I-Gel, a Single-Use Supraglottic Airway Device with a Non-Inflatable Cuff and an Esophageal Vent: An Observational Study in Children. Acta Anaesthesiol. Scand. 2009, 53, 376–379. [Google Scholar] [CrossRef]
- Theiler, L.; Gutzmann, M.; Kleine-Brueggeney, M.; Urwyler, N.; Kaempfen, B.; Greif, R. I-GelTM Supraglottic Airway in Clinical Practice: A Prospective Observational Multicentre Study. Br. J. Anaesth. 2012, 109, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Middleton, P.M.; Simpson, P.M.; Thomas, R.E.; Bendall, J.C. Higher Insertion Success with the I-Gel® Supraglottic Airway in out-of-Hospital Cardiac Arrest: A Randomised Controlled Trial. Resuscitation 2014, 85, 893–897. [Google Scholar] [CrossRef]
- Duckett, J.; Fell, P.; Han, K.; Kimber, C.; Taylor, C. Introduction of the I-Gel Supraglottic Airway Device for Prehospital Airway Management in a UK Ambulance Service. Emerg. Med. J. 2014, 31, 505–507. [Google Scholar] [CrossRef]
- Ruetzler, K.; Roessler, B.; Potura, L.; Priemayr, A.; Robak, O.; Schuster, E.; Frass, M. Performance and Skill Retention of Intubation by Paramedics Using Seven Different Airway Devices--a Manikin Study. Resuscitation 2011, 82, 593–597. [Google Scholar] [CrossRef]
- Benger, J.R.; Kirby, K.; Black, S.; Brett, S.J.; Clout, M.; Lazaroo, M.J.; Nolan, J.P.; Reeves, B.C.; Robinson, M.; Scott, L.J.; et al. Supraglottic Airway Device versus Tracheal Intubation in the Initial Airway Management of Out-of-Hospital Cardiac Arrest: The AIRWAYS-2 Cluster RCT. Health Technol. Assess 2022, 26, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.J.; Chantler, P.J.; Baskett, P.J. The Incidence of Regurgitation during Cardiopulmonary Resuscitation: A Comparison between the Bag Valve Mask and Laryngeal Mask Airway. Resuscitation 1998, 38, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Häske, D.; Schempf, B.; Gaier, G.; Niederberger, C. Performance of the I-GelTM during Pre-Hospital Cardiopulmonary Resuscitation. Resuscitation 2013, 84, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Stuby, L.; Jampen, L.; Sierro, J.; Paus, E.; Spichiger, T.; Suppan, L.; Thurre, D. Effect on Chest Compression Fraction of Continuous Manual Compressions with Asynchronous Ventilations Using an I-Gel® versus 30:2 Approach during Simulated Out-of-Hospital Cardiac Arrest: Protocol for a Manikin Multicenter Randomized Controlled Trial. Healthcare 2021, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Stuby, L.; Jampen, L.; Sierro, J.; Bergeron, M.; Paus, E.; Spichiger, T.; Suppan, L.; Thurre, D. Effect of Early Supraglottic Airway Device Insertion on Chest Compression Fraction during Simulated Out-of-Hospital Cardiac Arrest: Randomised Controlled Trial. J. Clin. Med. 2022, 11, 217. [Google Scholar] [CrossRef]
- Stuby, L.; Suppan, L.; Jampen, L.; Thurre, D. Impact of the Over-the-Head Position with a Supraglottic Airway Device on Chest Compression Depth and Rate: A Post Hoc Analysis of a Randomized Controlled Trial. Healthcare 2022, 10, 718. [Google Scholar] [CrossRef]
- Benger, J.R.; Lazaroo, M.J.; Clout, M.; Voss, S.; Black, S.; Brett, S.J.; Kirby, K.; Nolan, J.P.; Reeves, B.C.; Robinson, M.; et al. Randomized Trial of the I-Gel Supraglottic Airway Device versus Tracheal Intubation during out of Hospital Cardiac Arrest (AIRWAYS-2): Patient Outcomes at Three and Six Months. Resuscitation 2020, 157, 74–82. [Google Scholar] [CrossRef]
- Hansen, M.; Wang, H.; Le, N.; Lin, A.; Idris, A.; Kornegay, J.; Schmicker, R.; Daya, M. Prospective Evaluation of Airway Management in Pediatric Out-of-Hospital Cardiac Arrest. Resuscitation 2020, 156, 53–60. [Google Scholar] [CrossRef]
- Hanlin, E.R.; Chan, H.K.; Hansen, M.; Wendelberger, B.; Shah, M.I.; Bosson, N.; Gausche-Hill, M.; VanBuren, J.M.; Wang, H.E. Epidemiology of Out-of-Hospital Pediatric Airway Management in the 2019 National Emergency Medical Services Information System Data Set. Resuscitation 2022, 173, 124–133. [Google Scholar] [CrossRef]
- Mani, S.; Gugino, S.; Helman, J.; Bawa, M.; Nair, J.; Chandrasekharan, P.; Rawat, M. Laryngeal Mask Ventilation with Chest Compression during Neonatal Resuscitation: Randomized, Non-Inferiority Trial in Lambs. Pediatr. Res. 2021, 92, 671–677. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Rouzioux, J.; Montassier, E.; Baert, V.; Recher, M.; Hubert, H.; Leteurtre, S.; Javaudin, F. GR-RéAC Endotracheal Intubation versus Supraglottic Procedure in Paediatric Out-of-Hospital Cardiac Arrest: A Registry-Based Study. Resuscitation 2021, 168, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Li, T.; Altman, D.G.; Elbourne, D. CONSORT 2010 Statement: Extension to Randomised Crossover Trials. BMJ 2019, 366, l4378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.; Kessler, D.; Mackinnon, R.; Chang, T.P.; Nadkarni, V.M.; Hunt, E.A.; Duval-Arnould, J.; Lin, Y.; Cook, D.A.; Pusic, M.; et al. Reporting Guidelines for Health Care Simulation Research: Extensions to the CONSORT and STROBE Statements. Simul. Healthc. 2016, 11, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.-W.; Tetzlaff, J.M.; Gøtzsche, P.C.; Altman, D.G.; Mann, H.; Berlin, J.A.; Dickersin, K.; Hróbjartsson, A.; Schulz, K.F.; Parulekar, W.R.; et al. SPIRIT 2013 Explanation and Elaboration: Guidance for Protocols of Clinical Trials. BMJ 2013, 346, e7586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Medical Association. World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. J. Postgrad Med. 2001, 47, 199–203.
- Keamk—Create Random and Balanced Teams. Available online: https://www.keamk.com/ (accessed on 5 January 2021).
- Create a Blocked Randomisation List|Sealed Envelope. Available online: https://www.sealedenvelope.com/simple-randomiser/v1/lists (accessed on 6 December 2020).
- Suppan, L.; Jampen, L.; Siebert, J.N.; Zünd, S.; Stuby, L.; Ozainne, F. Impact of Two Resuscitation Sequences on Alveolar Ventilation during the First Minute of Simulated Pediatric Cardiac Arrest: Randomized Cross-Over Trial. Healthcare 2022, 10, 2451. [Google Scholar] [CrossRef]
- Tinning, K.; Acworth, J. Make Your Best Guess: An Updated Method for Paediatric Weight Estimation in Emergencies. Emerg. Med. Australas. 2007, 19, 528–534. [Google Scholar] [CrossRef]
- London.Mp3 by Walter_Odington. Available online: https://freesound.org/people/Walter_Odington/sounds/18021/ (accessed on 24 October 2022).
- Numa, A.H.; Newth, C.J. Anatomic Dead Space in Infants and Children. J. Appl. Physiol. 1996, 80, 1485–1489. [Google Scholar] [CrossRef]
- Di Nardo, M.; Grasso, S. Should We Set Tidal Volume in Children Using the Driving Pressure? Pediatr. Crit. Care Med. 2019, 20, 905. [Google Scholar] [CrossRef]
- Maconochie, I.K.; Aickin, R.; Hazinski, M.F.; Atkins, D.L.; Bingham, R.; Couto, T.B.; Guerguerian, A.-M.; Nadkarni, V.M.; Ng, K.-C.; Nuthall, G.A.; et al. Pediatric Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation 2020, 156, A120–A155. [Google Scholar] [CrossRef] [PubMed]
- Yareta—Portal. Available online: https://yareta.unige.ch/home (accessed on 29 October 2022).
- Morris, M.C.; Nadkarni, V.M.; Ward, F.R.; Nelson, R.M. Exception from Informed Consent for Pediatric Resuscitation Research: Community Consultation for a Trial of Brain Cooling after in-Hospital Cardiac Arrest. Pediatrics 2004, 114, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Gabbott, D.A.; Beringer, R. The IGEL Supraglottic Airway: A Potential Role for Resuscitation? Resuscitation 2007, 73, 161–162. [Google Scholar] [CrossRef] [PubMed]


| Study Period | ||||
|---|---|---|---|---|
| Enrolment | Intervention | Close-Out | ||
| Timepoint | t0 | t1 | t2 | |
| STUDY PROCEDURES | ||||
| Recruitment and consent | ✓ | |||
| Eligibility check | ✓ | |||
| Teams of two | ✓ | |||
| 30 min self-managed training | ✓ | |||
| ASSESSMENTS | ||||
| Participants’ characteristics | ✓ | |||
| Randomization | ✓ | |||
| Standard approach |  | ✓ | ||
| Experimental approach | ✓ | |||
| ✓: Performed | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuby, L.; Mühlemann, E.; Jampen, L.; Thurre, D.; Siebert, J.N.; Suppan, L. Effect of Intermediate Airway Management on Ventilation Parameters in Simulated Pediatric Out-of-Hospital Cardiac Arrest: Protocol for a Multicenter, Randomized, Crossover Trial. Children 2023, 10, 148. https://0-doi-org.brum.beds.ac.uk/10.3390/children10010148
Stuby L, Mühlemann E, Jampen L, Thurre D, Siebert JN, Suppan L. Effect of Intermediate Airway Management on Ventilation Parameters in Simulated Pediatric Out-of-Hospital Cardiac Arrest: Protocol for a Multicenter, Randomized, Crossover Trial. Children. 2023; 10(1):148. https://0-doi-org.brum.beds.ac.uk/10.3390/children10010148
Chicago/Turabian StyleStuby, Loric, Elisa Mühlemann, Laurent Jampen, David Thurre, Johan N. Siebert, and Laurent Suppan. 2023. "Effect of Intermediate Airway Management on Ventilation Parameters in Simulated Pediatric Out-of-Hospital Cardiac Arrest: Protocol for a Multicenter, Randomized, Crossover Trial" Children 10, no. 1: 148. https://0-doi-org.brum.beds.ac.uk/10.3390/children10010148