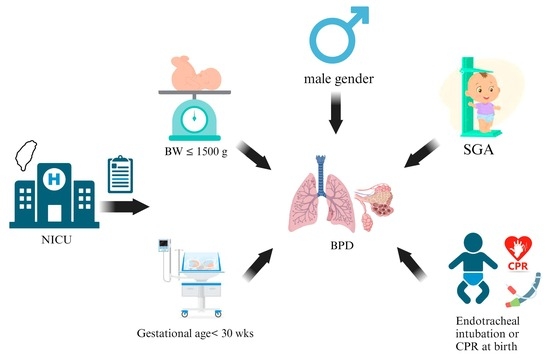
Comprehensive Analysis of Risk Factors for Bronchopulmonary Dysplasia in Preterm Infants in Taiwan: A Four-Year Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Outcome Measures and Variables
2.3. Statistics
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Risk Factors of Moderate-to-Severe BPD/Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPD | bronchopulmonary dysplasia |
| PMA | post-menstrual age |
| TNN | Taiwan Neonatal Network |
| NICU | neonatal intensive care unit |
| BW | birth weight |
| GA | gestational age |
| SGA | small for gestational age |
| AGA | average for gestational age |
| LGA | large for gestational age |
| IVH | intraventricular hemorrhage |
| RDS | respiratory distress syndrome |
| NO | inhaled nitric oxide |
| PDA | patent ductus arteriosus |
| NEC | necrotizing enterocolitis |
| HFO | high-frequency oscillatory ventilation |
| nCPAP | nasal continuous positive airway pressure |
| nIMV | nasal intermittent mandatory ventilation |
| nSIMV | nasal synchronized intermittent mandatory ventilation |
| SpO2 | oxygen saturation |
| NSD | normal spontaneous delivery |
| ETT | endotracheal intubation |
| CPCR | cardiopulmonary cerebral resuscitation |
References
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the nichd neonatal research network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef]
- Doyle, L.W.; Anderson, P.J. Long-term outcomes of bronchopulmonary dysplasia. Semin. Fetal Neonatal Med. 2009, 14, 391–395. [Google Scholar] [CrossRef]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef]
- Trembath, A.; Laughon, M.M. Predictors of bronchopulmonary dysplasia. Clin. Perinatol. 2012, 39, 585–601. [Google Scholar] [CrossRef]
- Walsh, M.C.; Yao, Q.; Gettner, P.; Hale, E.; Collins, M.; Hensman, A.; Everette, R.; Peters, N.; Miller, N.; Muran, G.; et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004, 114, 1305–1311. [Google Scholar] [CrossRef]
- Bose, C.; Van Marter, L.J.; Laughon, M.; O’Shea, T.M.; Allred, E.N.; Karna, P.; Ehrenkranz, R.A.; Boggess, K.; Leviton, A.; Extremely Low Gestational Age Newborn Study Investigators. Fetal growth restriction and chronic lung disease among infants born b efore the 28th week of gestation. Pediatrics 2009, 124, e450–e458. [Google Scholar] [CrossRef] [PubMed]
- Hartling, L.; Liang, Y.; Lacaze-Masmonteil, T. Chorioamnionitis as a risk factor for bronchopulmonary dysplasia: A systematic review and meta-analysis. Arch. Dis. Childhood. Fetal Neonatal Ed. 2012, 97, F8–F17. [Google Scholar] [CrossRef]
- McEvoy, C.T.; Spindel, E.R. Pulmonary effects of maternal smoking on the fetus and child: Effects on lung development, respiratory morbidities, and life long lung health. Paediatr. Respir. Rev. 2017, 21, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.A.; Lindstrom, D.P.; Cotton, R.B. Evidence from twin study implies possible genetic susceptibility to bronchopulmonary dysplasia. Semin. Perinatol. 1996, 20, 206–209. [Google Scholar] [CrossRef]
- Coalson, J.J. Pathology of new bronchopulmonary dysplasia. Semin. Neonatol. 2003, 8, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kalikkot Thekkeveedu, R.; Guaman, M.C.; Shivanna, B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir. Med. 2017, 132, 170–177. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Shennan, A.T.; Dunn, M.S.; Ohlsson, A.; Lennox, K.; Hoskins, E.M. Abnormal pulmonary outcomes in premature infants: Prediction from oxygen requirement in the neonatal period. Pediatrics 1988, 82, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary dysplasia: Executive summary of a workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Walsh, M.C.; Vohr, B.R.; Jobe, A.H.; Wright, L.L.; Fanaroff, A.A.; Wrage, L.A.; Poole, K.; National Institutes of Child Health; Human Development Neonatal Research Network. Validation of the national institutes of health consensus definition o f bronchopulmonary dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef]
- Kurihara, C.; Zhang, L.; Mikhael, M. Newer bronchopulmonary dysplasia definitions and prediction of health economics impacts in very preterm infants. Pediatr. Pulmonol. 2021, 56, 409–417. [Google Scholar] [CrossRef]
- Kim, F.; Bateman, D.A.; Goldshtrom, N.; Sahni, R.; Wung, J.T.; Wallman-Stokes, A. Revisiting the definition of bronchopulmonary dysplasia in premature i nfants at a single center quaternary neonatal intensive care unit. J. Perinatol. 2021, 41, 756–763. [Google Scholar] [CrossRef]
- Lapcharoensap, W.; Gage, S.C.; Kan, P.; Profit, J.; Shaw, G.M.; Gould, J.B.; Stevenson, D.K.; O’Brodovich, H.; Lee, H.C. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015, 169, e143676. [Google Scholar] [CrossRef]
- Keszler, M.; Sant’Anna, G. Mechanical ventilation and bronchopulmonary dysplasia. Clin. Perinatol. 2015, 42, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Geetha, O.; Rajadurai, V.S.; Anand, A.J.; Dela Puerta, R.; Huey Quek, B.; Khoo, P.C.; Chua, M.C.; Agarwal, P. New bpd-prevalence and risk factors for bronchopulmonary dysplasia/mortality in extremely low gestational age infants ≤28 weeks. J. Perinatol. 2021, 41, 1943–1950. [Google Scholar] [CrossRef]
- Jobe, A.J. The new bpd: An arrest of lung development. Pediatr. Res. 1999, 46, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Zysman-Colman, Z.; Tremblay, G.M.; Bandeali, S.; Landry, J.S. Bronchopulmonary dysplasia—Trends over three decades. Paediatr. Child. Health 2013, 18, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, Q.; Wang, S.; Dong, T.; Liang, L.; Xu, F.; He, Y.; Li, C.; Luo, F.; Liang, J.; et al. Risk factors that affect the degree of bronchopulmonary dysplasia in v ery preterm infants: A 5-year retrospective study. BMC Pediatr. 2022, 22, 200. [Google Scholar] [CrossRef]
- Ambalavanan, N.; Van Meurs, K.P.; Perritt, R.; Carlo, W.A.; Ehrenkranz, R.A.; Stevenson, D.K.; Lemons, J.A.; Poole, W.K.; Higgins, R.D.; NICHD Neonatal Research Network. Predictors of death or bronchopulmonary dysplasia in preterm infants with respiratory failure. J. Perinatol. 2008, 28, 420–426. [Google Scholar] [CrossRef]
- Zhu, Z.; Yuan, L.; Wang, J.; Li, Q.; Yang, C.; Gao, X.; Chen, S.; Han, S.; Liu, J.; Wu, H.; et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in china from 2010 to 2019. JAMA Netw. Open 2021, 4, e219382. [Google Scholar] [CrossRef]
- Reiss, I.; Landmann, E.; Heckmann, M.; Misselwitz, B.; Gortner, L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch. Gynecol. Obstet. 2003, 269, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Kato, S.; Saito, M.; Miyahara, N.; Arai, H.; Namba, F.; Ota, E.; Nakanishi, H. Bronchopulmonary dysplasia in extremely premature infants: A scoping review for identifying risk factors. Biomedicines 2023, 11, 553. [Google Scholar] [CrossRef]
- Chien, L.Y.; Whyte, R.; Thiessen, P.; Walker, R.; Brabyn, D.; Lee, S.K.; Canadian Neonatal Network. Snap-ii predicts severe intraventricular hemorrhage and chronic lung disease in the neonatal intensive care unit. J. Perinatol. 2002, 22, 26–30. [Google Scholar] [CrossRef]

| Total (N = 3111) | N (%) |
|---|---|
| Gestational age, Mean ± SD | 27.5 ± 2.0 |
| <28 week | 1405 (45.2) |
| ≥28 week | 1706 (54.8) |
| Sex | |
| Male | 1679 (54.0) |
| Female | 1432 (46.0) |
| SGA/AGA/LGA | |
| Small for gestational age | 738 (23.7) |
| Appropriate for gestational age | 2342 (75.3) |
| Large for gestational age | 31 (1.0) |
| Birth weight (g) | |
| <501 | 51 (1.6) |
| 501–600 | 122 (3.9) |
| 601–700 | 281 (9.0) |
| 701–800 | 321 (10.3) |
| 801–900 | 364 (11.7) |
| 901–1000 | 360 (11.6) |
| 1001–1100 | 355 (11.4) |
| 1101–1200 | 340 (10.9) |
| 1201–1300 | 293 (9.4) |
| 1301–1400 | 314 (10.1) |
| 1401–1500 | 249 (8.0) |
| >1500 g | 61 (2.0) |
| Delivery | |
| Vaginal delivery | 999 (32.1) |
| Cesarean section | 2093 (67.3) |
| Multiple births | |
| No | 2233 (71.8) |
| Yes | 878 (28.2) |
| N = 3111 | Non-BPD (n = 1153) | BPD (n = 1958) | p Value |
|---|---|---|---|
| GA, mean ± SD | 28.70 ± 1.235 | 26.76 ± 1.974 | <0.001 *** |
| Taiwan nationality, n (%) | 1123 (37.2) | 1898 (62.8) | 0.265 |
| Multiple births, n (%) | 336 (38.3) | 542 (61.7) | 0.202 |
| NSD, n (%) | 330 (33.0) | 669 (67.0) | 0.001 ** |
| Maternal ANS, n (%) | 997 (37.4) | 1672 (62.6) | 0.152 |
| Maternal MgSO4 use, n (%) | 670 (37.6) | 1112 (62.4) | 0.242 |
| Chorioamnionitis, n (%) | 135 (23.7) | 435 (76.3) | <0.001 *** |
| Non-PIH, n (%) | 854 (35.3) | 1563 (64.7) | <0.001 *** |
| Low BBW (≤1000 g), n (%) | 274 (18.3) | 1225 (81.7) | <0.001 *** |
| Male gender, n (%) | 603 (35.9) | 1076 (64.1) | 0.081 |
| BBW for GA, n (%) | 0.318 | ||
| SGA | 290 (39.3) | 448 (60.7) | |
| AGA | 853 (36.4) | 1489 (63.6) | |
| LGA | 10 (32.3) | 21 (67.7) | |
| 5 minute Apgar score, n (%) | <0.001 *** | ||
| 0–6 | 114 (20.8) | 435 (79.2) | |
| 7–10 | 1035 (40.6) | 1517 (59.4) | |
| Oxygen or face mask use in initial resuscitation, n (%) | 1069 (36.0) | 1901 (64.0) | <0.001 *** |
| ETT or CPCR in initial resuscitation, n (%) | 177 (16.1) | 925 (83.9) | <0.001 *** |
| Non-nCPAP in initial resuscitation, n (%) | 738 (34.0) | 1432 (66.0) | <0.001 *** |
| Bacteremia before 3 days old, n (%) | 31 (31.6) | 67 (68.4) | 0.153 |
| RDS, n (%) | 864 (34.4) | 1647 (65.6) | <0.001 *** |
| Oxygen use during hospitalization | 972 (33.3) | 1951 (66.7) | <0.001 *** |
| ETT+MV or ETT+HFO during hospitalization, n (%) | 353 (19.1) | 1499 (80.9) | <0.001 *** |
| Nasal high-flow canula or nIMV or nSIMV during hospitalization, n (%) | 741 (32.1) | 1566 (67.9) | <0.001 *** |
| Non-nCPAP use during hospitalization | 26 (20.8) | 99 (79.2) | <0.001 *** |
| Non-nCPAP use before ETT, n (%) | 139 (16.3) | 714 (83.7) | <0.001 *** |
| Surfactant therapy in initial resuscitation, n (%) | 1 (16.7) | 5 (83.3) | 0.281 |
| Surfactant therapy during hospitalization, n (%) | 293 (21.5) | 1071 (78.5) | <0.001 *** |
| Inhaled NO therapy, n (%) | 10 (3.6) | 269 (96.4) | <0.001 *** |
| Pneumothorax | 28 (18.7) | 122 (81.3) | <0.001 *** |
| Sepsis or meningitis, n (%) | 46 (13.3) | 301 (86.7) | <0.001 *** |
| IVH, n (%) | <0.001 *** | ||
| 0–2 | 1117 (39.0) | 1744 (61.0) | |
| 3–4 | 35 (14.3) | 210 (85.7) | |
| PDA, n (%) | 435 (25.9) | 1246 (74.1) | <0.001 *** |
| NEC, n (%) | 45 (23.8) | 144 (76.2) | <0.001 *** |
| Death, n (%) | 3 (3.1) | 94 (96.9) | <0.001 *** |
| N = 3111 | OR (95% CI) | p Value |
|---|---|---|
| Low GA | 0.661 (0.604~0.723) | <0.001 *** |
| Multiple births | 1.152 (0.921~1.440) | 0.216 |
| Cesarean section | 0.813 (0.642~1.029) | 0.085 |
| Maternal ANS | 0.984 (0.721~1.342) | 0.919 |
| Maternal MgSO4 use | 0.839 (0.677~1.040) | 0.110 |
| Chorioamnionitis | 1.781 (1.347~2.356) | <0.001 *** |
| Maternal PIH | 0.969 (0.747~1.258) | 0.814 |
| Low BBW (≦1000 g) | 1.675 (1.245~2.252) | 0.001 ** |
| Male gender | 1.143 (0.941~1.387) | 0.178 |
| BBW for GA | 0.681 | |
| SGA | – | |
| AGA | 0.893 (0.666~1.197) | |
| LGA | 0.719 (0.270~1.920) | |
| Five minute Apgar score | 0.042 * | |
| 0–6 | – | |
| 7–10 | 1.398 (1.012~1.932) | |
| Oxygen or face mask use in initial resuscitation | 1.255 (0.735~2.144) | 0.405 |
| ETT or CPCR in initial resuscitation | 1.305 (0.965~1.765) | 0.084 |
| nCPAP in initial resuscitation | 0.925 (0.740~1.156) | 0.491 |
| Bacteremia before 3 days old | 0.651 (0.353~1.199) | 0.169 |
| RDS | 0.687 (0.528~0.894) | 0.005 ** |
| Oxygen use during hospitalization | 18.334 (7.951~42.275) | <0.001 *** |
| ETT + MV or ETT + HFO during hospitalization | 2.315 (1.785~3.003) | <0.001 *** |
| Nasal high-flow canula or nIMV or nSIMV during hospitalization | 1.762 (1.393~2.229) | <0.001 *** |
| nCPAP during hospitalization | 0.870 (0.446~1.695) | 0.682 |
| Surfactant therapy in initial resuscitation | 1.646 (0.140~19.363) | 0.692 |
| Surfactant therapy during hospitalization | 1.173 (0.913~1.508) | 0.212 |
| Inhaled NO therapy | 5.461 (2.531~11.783) | <0.001 *** |
| Pneumothorax | 0.945 (0.547~1.633) | 0.840 |
| Sepsis or meningitis | 2.314 (1.578~3.392) | <0.001 *** |
| IVH | 0.480 | |
| 0–2 | – | |
| 3–4 | 1.179 (0.746~1.865) | |
| PDA | 1.295 (1.051~1.596) | 0.015 * |
| NEC | 0.937 (0.592~1.485) | 0.783 |
| Moderate-to-Severe BPD n = 2594 | Moderate-to-Severe BPD/Death before 28 Days Old n = 3016 | Moderate-to-Severe BPD/Death before 36 Weeks PMA n = 3067 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Low GA | 0.612 (0.551~0.679) | <0.001 *** | 0.609 (0.549~0.675) | <0.001 *** | 0.612 (0.552~0.678) | <0.001 *** |
| Multiple births | 1.245 (0.956~1.620) | 0.103 | 1.225 (0.944~1.588) | 0.127 | 1.221 (0.942~1.583) | 0.132 |
| Cesarean section | 0.801 (0.604~1.062) | 0.123 | 0.806 (0.610~1.065) | 0.129 | 0.794 (0.602~1.049) | 0.105 |
| Maternal ANS | 1.256 (0.867~1.820) | 0.228 | 1.189 (0.826~1.713) | 0.352 | 1.213 (0.843~1.747) | 0.298 |
| Maternal MgSO4 use | 0.812 (0.630~1.045) | 0.106 | 0.841 (0.655~1.078) | 0.172 | 0.839 (0.655~1.076) | 0.167 |
| Chorioamnionitis | 1.686 (1.216~2.338) | 0.002 ** | 1.642 (1.188~2.268) | 0.003 ** | 1.613 (1.168~2.226) | 0.004 ** |
| Maternal PIH | 0.950 (0.699~1.291) | 0.744 | 0.932 (0.689~1.260) | 0.646 | 0.931 (0.689~1.259) | 0.643 |
| Low BBW (≦1000 g) | 2.049 (1.473~2.857) | <0.001 *** | 1.992 (1.433~2.762) | <0.001 *** | 1.992 (1.435~2.762) | <0.001 *** |
| Male gender | 1.259 (1.002~1.582) | 0.048 * | 1.245 (0.994~1.560) | 0.057 | 1.224 (0.978~1.531) | 0.079 |
| BBW for GA | 0.023 * | 0.016 * | 0.018 ** | |||
| SGA | – | – | – | |||
| AGA | 0.620 (0.441~0.871) | 0.611 (0.437~0.856) | 0.616 (0.440~0.861) | |||
| LGA | 0.578 (0.190~1.757) | 0.545 (0.180~1.648) | 0.545 (0.180~1.647) | |||
| Five minute Apgar score | 0.056 | 0.093 | 0.099 | |||
| 0–6 | – | – | – | |||
| 7–10 | 1.412 (0.990~2.013) | 1.349 (0.951~1.912) | 1.341 (0.946~1.900) | |||
| Oxygen or face mask use in initial resuscitation | 1.343 (0.698~2.584) | 0.377 | 1.372 (0.721~2.610) | 0.336 | 1.377 (0.723~2.621) | 0.331 |
| ETT or CPCR in initial resuscitation | 1.499 (1.074~2.091) | 0.017 * | 1.449 (1.042~2.014) | 0.028 * | 1.412 (1.016~1.963) | 0.040 * |
| nCPAP in initial resuscitation | 1.055 (0.809~1.375) | 0.695 | 1.052 (0.809~1.370) | 0.704 | 1.047 (0.804~1.361) | 0.735 |
| Bacteremia before 3 days old | 0.526 (0.259~1.069) | 0.076 | 0.541 (0.272~1.077) | 0.808 | 0.605 (0.305~1.197) | 0.149 |
| RDS | 0.788 (0.567~1.095) | 0.156 | 0.777 (0.562~1.075) | 0.127 | 0.769 (0.557~1.062) | 0.111 |
| Oxygen use during hospitalization | 17.999 (5.660~57.236) | <0.001 *** | 10.593 (4.508~24.895) | <0.001 *** | 10.882 (4.618~25.643) | <0.001 *** |
| ETT + MV or ETT + HFO during hospitalization | 3.097 (2.308~4.155) | <0.001 *** | 3.112 (2.325~4.165) | <0.001 *** | 3.219 (2.407~4.305) | <0.001 *** |
| Nasal high-flow canula or nIMV or nSIMV during hospitalization | 2.046 (1.536~2.724) | <0.001 *** | 1.866 (1.408~2.474) | <0.001 *** | 1.804 (1.363~2.388) | <0.001 *** |
| nCPAP during hospitalization | 1.437 (0.641~3.221) | 0.379 | 0.528 (0.265~1.051) | 0.069 | 0.506 (0.256~1.000) | 0.050 |
| Surfactant therapy in initial resuscitation | 3.040 (0.237~38.932) | 0.393 | 2.613 (0.179~38.082) | 0.482 | 2.663 (0.184~38.517) | 0.472 |
| Surfactant therapy during hospitalization | 1.156 (0.870~1.536) | 0.318 | 1.164 (0.880~1.540) | 0.287 | 1.143 (0.864~1.511) | 0.349 |
| Inhaled NO therapy | 6.505 (2.945~14.370) | <0.001 *** | 7.197 (3.306~15.669) | <0.001 *** | 7.201 (3.317~15.633) | <0.001 *** |
| Pneumothorax | 0.977 (0.540~1.771) | 0.940 | 1.147 (0.638~2.065) | 0.646 | 1.128 (0.628~2.025) | 0.687 |
| Sepsis/meningitis | 2.651 (1.726~4.071) | <0.001 *** | 2.887 (1.887~4.418) | <0.001 *** | 2.855 (1.868~4.363) | <0.001 *** |
| IVH | 0.781 | 0.269 | 0.155 | |||
| 0–2 | – | – | – | |||
| 3–4 | 1.073 (0.653~1.765) | 1.318 (0.808~2.152) | 1.424 (0.875~2.318) | |||
| PDA | 1.589 (1.249~2.022) | <0.001 *** | 1.541 (1.216~1.954) | <0.001 *** | 1.556 (1.228~1.972) | <0.001 *** |
| NEC | 0.921 (0.547~1.551) | 0.758 | 1.064 (0.636~1.779) | 0.814 | 1.083 (0.650~1.805) | 0.759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-Y.; Lin, T.-I.; Lin, C.-H.; Yang, S.-N.; Chen, W.-J.; Wu, C.-Y.; Liu, H.-K.; Wu, P.-L.; Suen, J.-L.; Chen, J.-S.; et al. Comprehensive Analysis of Risk Factors for Bronchopulmonary Dysplasia in Preterm Infants in Taiwan: A Four-Year Study. Children 2023, 10, 1822. https://0-doi-org.brum.beds.ac.uk/10.3390/children10111822
Huang L-Y, Lin T-I, Lin C-H, Yang S-N, Chen W-J, Wu C-Y, Liu H-K, Wu P-L, Suen J-L, Chen J-S, et al. Comprehensive Analysis of Risk Factors for Bronchopulmonary Dysplasia in Preterm Infants in Taiwan: A Four-Year Study. Children. 2023; 10(11):1822. https://0-doi-org.brum.beds.ac.uk/10.3390/children10111822
Chicago/Turabian StyleHuang, Lin-Yi, Ting-I Lin, Chyi-Her Lin, San-Nan Yang, Wan-Ju Chen, Chien-Yi Wu, Hsien-Kuan Liu, Pei-Ling Wu, Jau-Ling Suen, Jung-Sheng Chen, and et al. 2023. "Comprehensive Analysis of Risk Factors for Bronchopulmonary Dysplasia in Preterm Infants in Taiwan: A Four-Year Study" Children 10, no. 11: 1822. https://0-doi-org.brum.beds.ac.uk/10.3390/children10111822