Gastroschisis: A State-of-the-Art Review
Abstract
:1. Introduction
2. Definition
3. Pathogenesis
4. Epidemiology
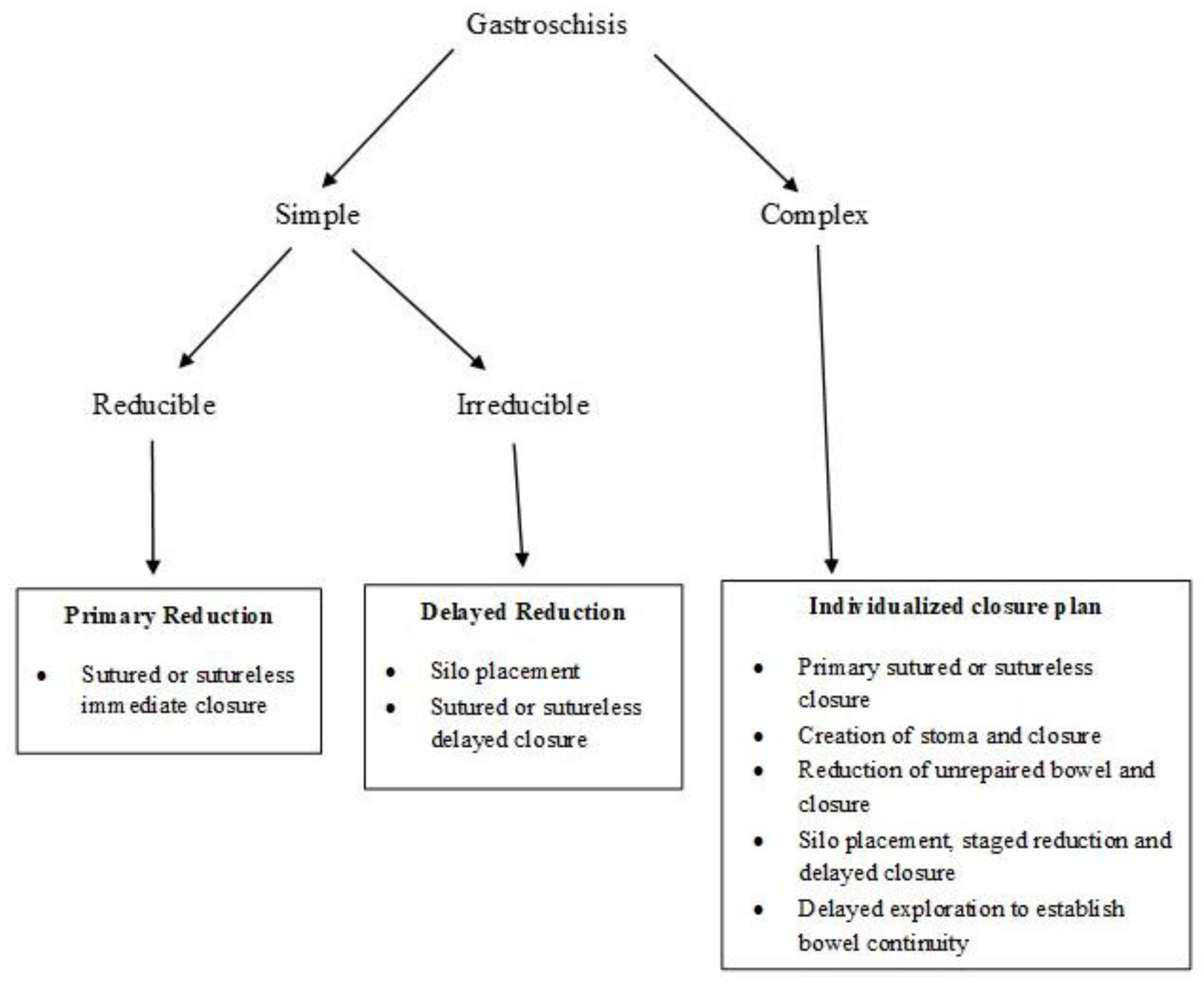
5. Types of Gastroschisis
6. Prenatal Diagnosis
7. Management during Pregnancy
8. Timing and Mode of Delivery
9. Postnatal Management
10. Surgical Management
- (a)
- Primary reduction, with either immediate sutured closure or sutureless closure;
- (b)
- Prosthetic silo placement, gradual visceral reduction followed by delayed sutured or sutureless closure.
10.1. Primary Reduction
10.2. Staged Reduction
10.3. Sutureless Closure
10.4. Primary Reduction vs. Staged Reduction
10.5. Sutured Closure vs. Sutureless Closure
11. Postoperative Management
12. Prognosis and Outcome Prediction
13. Medium- and Long-Term Outcomes
14. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Opitz, J.M.; Feldkamp, M.L.; Botto, L.D. An evolutionary and developmental biology approach to gastroschisis. Birth Defects Res. 2019, 111, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.C.; Stokes, G.E. Gastroschisis; Report of two cases treated by a modification of the gross operation for omphalocele. Surgery 1953, 33, 112–120. [Google Scholar] [PubMed]
- International Clearinghouse for Birth Defects Surveillance and Research. Annual Report 2014. International Centre on Birth Defects (ICBDSR Centre), Rome, Italy. Available online: http://www.icbdsr.org/wp-content/annual_report/Report2014.pdf (accessed on 4 August 2020).
- Abdullah, F.; Arnold, M.A.; Nabaweesi, R.; Fischer, A.C.; Colombani, P.M.; Anderson, K.D.; Lau, H.; Chang, D.C. Gastroschisis in the United States 1988–2003: Analysis and risk categorization of 4344 patients. J. Prinatol. 2007, 27, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledbetter, D.J. Gastroschisis and omphalocele. Surg. Clin. N. Am. 2006, 86, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, B. Embryology of exomphalos and allied malformations. Arch. Dis. Child. 1963, 38, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, A. The myth of gastroschisis. J. Pediatr. Surg. 1975, 10, 235–244. [Google Scholar] [CrossRef]
- De Vries, P.A. The pathogenesis of gastroschisis and omphalocele. J. Pediatr. Surg. 1980, 15, 245–251. [Google Scholar] [CrossRef]
- Hoyme, H.E.; Higginbottom, M.C.; Jones, K.L. The vascular pathogenesis of gastroschisis; intrauterine interruption of the omphalomesenteric artery. J. Pediatr. 1981, 98, 228–231. [Google Scholar] [CrossRef]
- Feldkamp, M.L.; Carey, J.C.; Sadler, T.W. Development of gastroschisis: Review of hypotheses, a novel hypothesis, and implications for research. Am. J. Med. Genet. 2007, 143A, 639–652. [Google Scholar] [CrossRef]
- Stevenson, R.E.; Rogers, R.C.; Chandler, J.C.; Gauderer, M.W.L.; Hunter, A.G.W. Escape of the yolk sac: A hypothesis to explain the embryogenesis of gastroschisis. Cin. Genet. 2009, 75, 326–333. [Google Scholar] [CrossRef]
- Bargy, F.; Beaudoin, S. Comprehensive developmental mechanisms in Gastroschisis. Fetal Diagn. Ther. 2014, 36, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lubinsky, M. A vascular and thrombotic model of gastroschisis. Am. J. Med. Genet. 2014, 164A, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Rittler, M.; Vauthay, L.; Mazzitelli, N. Gastroschisis is a defect of the umbilical ring: Evidence from morphological evaluation of stillborn fetuses. Birth Defects Res. A Clin. Mol. Teratol. 2013, 97, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, S. Insights into the etiology and embryology of gastroschisis. Semin. Pediatr. Surg. 2018, 27, 283–288. [Google Scholar] [CrossRef]
- Kirby, R.S.; Marshall, J.; Tanner, J.P.; Salemi, J.L.; Feldkamp, M.L.; Marengo, L.; Meyer, R.E.; Druschel, C.M.; Rickard, R.; Kucik, J.E.; et al. Prevalence and correlates of gastroschisis in 15 states, 1995 to 2005. Obstetr. Gynecol. 2013, 122, 275–281. Available online: https://journals.lww.com/greenjournal/Fulltext/2013/08000/Prevalence_and_Correlates_of_Gastroschisis_in_15.14.aspx (accessed on 4 August 2020). [CrossRef] [Green Version]
- Castilla, E.E.; Mastroiacovo, P.; Orioli, I.M. Gastroschisis: International Epidemiology and Public Health Perspectives. Amer. J. Med. Genet. Part C Semin. Med. Genet. 2008, 148C, 162–179. [Google Scholar] [CrossRef]
- Short, T.D.; Stallings, E.B.; Isenburg, J.; O’Leary, L.A.; Yazdy, M.M.; Bohm, M.K.; Ethen, M.; Chen, X.; Tran, T.; Fox, D.J.; et al. Gastroschisis trends and ecologic link to opioid prescription rates—United States, 2006–2015. Morb. Mortal. Wkly. Rep. 2019, 68, 31–36. [Google Scholar] [CrossRef]
- Loane, M.; Dolk, H.; Bradbury, I.; EUROCAT Working Group. Increasing prevalence of gastroschisis in Europe 1980–2002; a phenomenon restricted to younger mothers? Pediatr. Perinat. Epidemiol. 2007, 21, 363–369. [Google Scholar] [CrossRef]
- Skarsgard, E.D.; Meaney, C.; Bassil, K.; Brindle, M.; Arbour, L.; Moineddin, R.; Canadian Pediatric Surgery Network (CAPSNet). Maternal Risk Factors for Gastroschisis in Canada. Birth Def. Res. 2015, 103, 111–118. [Google Scholar] [CrossRef]
- Jones, A.M.; Isenburg, J.; Salemi, J.L.; Arnold, K.E.; Mai, C.T.; Aggarwal, D.; Arias, W.; Carrino, G.E.; Ferrell, E.; Folorunso, O.; et al. Increasing Prevalence of Gastroschisis—14 States, 1995–2012. Morb. Mortal. Wkly. Rep. 2016, 65, 23–26. [Google Scholar] [CrossRef] [Green Version]
- Stallings, E.B.; Isenburg, J.L.; Short, T.D.; Heinke, D.; Kirby, R.S.; Romitti, P.A.; Canfield, M.A.; O’Leary, L.A.; Liberman, R.F.; Forestieri, N.E.; et al. Population-based birth defects in the United States, 2012–2016: A focus on abdominal wall defects. Birth Def. Res. 2019, 111, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, S.; Santoro, M.; Coi, A.; Mezzasalma, L.; Bianchi, F.; Pierini, A. Lifestyle and sociodemographic risk factors for gastroschisis; a systematic review and meta-analysis. Arch. Dis. Child. 2020, 105, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Chabra, S.; Gleason, C.A.; Seidel, K.; Williams, M.A. Rising Prevalence of Gastroschisis in Washington State. J. Toxicol. Environ. Health. A 2011, 74, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.P.; Langlois, P.H.; Suarez, L.; Brender, J.; Shanmugam, R. Association of Paternal Age with Prevalence of Selected Birth Defects. Birth Defects Res. A Clin. Mol. Teratol. 2007, 79, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Green, R.F.; Devine, O.; Crider, K.S.; Olney, R.S.; Archer, N.; Olshan, A.F.; Shapira, S.K.; National Birth Defects Prevention Study. Association of Paternal Age and Risk for Major Congenital Anomalies from the National Birth Defects Prevention Study, 1997 to 2004. Ann. Epidemiol. 2010, 20, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazaura, M.R.; Lie, R.T.; Irgens, L.M.; Didriksen, A.; Kapstad, M.; Egenaes, J.; Bjerkedal, T. Increasing Risk of Gastroschisis in Norway: An Age-Period-Cohort Analysis. Am. J. Epidemiol. 2004, 159, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldkamp, M.L.; Arnold, K.E.; Krikov, S.; Reefhuis, J.; Almli, L.M.; Moore, C.A.; Botto, L.D. Risk of gastroschisis with maternal gentiourinary infections: The US National birth defects prevention study 1997–2011. BMJ Open 2019, 9, e026297. [Google Scholar] [CrossRef]
- Root, E.D.; Meyer, R.E.; Emch, M.E. Evidence of Localized Clustering of Gastroschisis Births in North Carolina, 1999–2004. Soc. Sci. Med. 2009, 68, 1361–1367. [Google Scholar] [CrossRef]
- Bassil, K.L.; Yang, J.; Arbour, L.; Moineddin, R.; Brindle, M.E.; Hazell, E.; Skarsgard, E.D. Spatial variability of gastroschisis in Canada, 2006–2011: An exploratory analysis. Can. J. Public Health 2016, 107, e62–e67. [Google Scholar] [CrossRef]
- Waller, S.A.; Paul, K.; Peterson, S.E.; Hitti, J.E. Agricultural-related Chemical Exposures, Season of Conception, and Risk of Gastroschisis in Washington State. Am. J. Obstet. Gynecol. 2010, 202, 241.e1–241.e6. [Google Scholar] [CrossRef]
- Souther, C.; Puapong, D.P.; Woo, R.; Johnson, S.M. Possible etiologies of increased incidence of gastroschisis. Pediatr. Surg. Int. 2017, 33, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Molik, K.A.; Gingalewski, C.A.; West, K.W.; Rescorla, F.J.; Scherer, L.R.; Engum, S.A.; Grosfeld, J.L. Gastroschisis: A Plea for Risk Categorization. J. Pediatr. Surg. 2001, 36, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Perrone, E.E.; Olson, J.; Golden, J.M.; Besner, G.E.; Gayer, C.P.; Islam, S.; Gollin, G. Closing Gastroschisis: The Good, the Bad, and the Not-So Ugly. J. Pediatr. Surg. 2019, 54, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Bergholz, R.; Boettcher, M.; Reinshagen, K.; Wenke, K. Complex Gastroschisis Is a Different Entity to Simple Gastroschisis Affecting Morbidity and Mortality—A Systematic Review and Meta-Analysis. J. Pediatr. Surg. 2014, 49, 1527–1532. [Google Scholar] [CrossRef]
- Suver, D.; Lee, S.L.; Shekherdimian, S.; Kim, S.S. Left-sided Gastroschisis: Higher Incidence of Extraintestinal Congenital Anomalies. Am. J. Surg. 2008, 195, 663–666. [Google Scholar] [CrossRef]
- AIUM Practice Parameter for the Performance of Detailed Second- and Third-Trimester Diagnostic Obstetric Ultrasound Examinations. J. Ultrasound Med. 2019, 38, 3093–3100. [CrossRef]
- Torres, U.S.; Portela-Oliveira, E.; Braga, F.D.C.B.; Werner, H., Jr.; Daltro, P.A.N.; Souza, A.S. When closure fails: What the Radiologist needs to know about the embryology, anatomy, and prenatal imaging of ventral body wall defects. Semin. Ultrasound CT MRI 2015, 36, 522–536. [Google Scholar] [CrossRef]
- Mastroiacovo, P.; Lisi, A.; Castilla, E.E.; Martínez-Frías, M.-L.; Bermejo, E.; Marengo, L.; Kucik, J.; Siffel, C.; Halliday, J.; Gatt, M.; et al. Gastroschisis and associated defects: An international study. Am. J. Med. Genet. Part A 2007, 143A, 660–671. [Google Scholar] [CrossRef]
- Raitio, A.; Lahtinen, A.; Syvänen, J.; Kemppainen, T.; Löyttyniemi, E.; Gissler, M.; Hyvärinen, A.; Helenius, I. Gastroschisis in Finland 1993 to 2014—Increasing Prevalence, High Rates of Abortion, and Survival: A Population-Based Study. Eur. J. Pediatr. Surg. 2020, 30, 536–540. [Google Scholar] [CrossRef]
- Akhtar, J.; Skarsgard, E.D.; Canadian Pediatric Surgery Network (CAPSNet). Associated malformations and the “hidden mortality” of gastroschisis. J. Pediatr. Surg. 2012, 47, 911–916. [Google Scholar] [CrossRef]
- Fisher, J.E.; Tolcher, M.C.; Shamshirsaz, A.A.; Espinoza, J.; Sanz Cortes, M.; Donepudi, R.; Belfort, M.A.; Nassr, A.A. Accuracy of Ultrasound to Predict Neonatal Birth Weight Among Fetuses with Gastroschisis: Impact on Timing of Delivery. J. Ultrasound Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, G.E.; Hill, L.E.; Knight, G.J.; Haddow, J.E.; Carpenter, M. Second-trimester Maternal Serum Alpha-Fetoprotein Levels in Pregnancies Associated with Gastroschisis and Omphalocele. Obstet. Gynecol. 1988, 71, 906–909. [Google Scholar] [PubMed]
- D’Antonio, F.; Virgone, C.; Rizzo, G.; Khalil, A.; Baud, D.; Cohen-Overbeek, T.E.; Kuleva, M.; Salomon, L.J.; Flacco, M.E.; Manzoli, L.; et al. Prenatal Risk Factors and Outcomes in Gastroschisis: A Meta-Analysis. Pediatrics 2015, 136, e159–e169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oakes, M.C.; Porto, M.; Chung, J.H. Advances in Prenatal and Perinatal Diagnosis and Management of Gastroschisis. Semin. Pediatr. Surg. 2018, 27, 289–299. [Google Scholar] [CrossRef]
- Horton, A.L.; Powell, M.S.; Wolfe, H.M. Intrauterine Growth Patterns in Fetal Gastroschisis. Am. J. Perinatol. 2010, 27, 211–217. [Google Scholar] [CrossRef]
- Netta, D.A.; Wilson, R.D.; Visintainer, P.; Johnson, M.P.; Hedrick, H.L.; Flake, A.W.; Adzick, N.S. Gastroschisis: Growth patterns and a proposed prenatal surveillance protocol. Fetal Diagn. Ther. 2007, 22, 352–357. [Google Scholar] [CrossRef]
- Payne, N.R.; Simonton, S.C.; Olsen, S.; Arnesen, M.A.; Pfleghaar, K.M. Growth Restriction in Gastroschisis: Quantification of Its Severity and Exploration of a Placental Cause. BMC Pediatr. 2011, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Carroll, S.G.; Kuo, P.Y.; Kyle, P.M.; Soothill, P.W. Fetal protein loss in gastroschisis as an explanation of associated morbidity. Am. J. Obstet. Gynecol. 2001, 184, 1297–1301. [Google Scholar] [CrossRef]
- Girsen, A.I.; Do, S.; Davis, A.S.; Hintz, S.R.; Desai, A.K.; Mansour, T.; Merritt, T.A.; Oshiro, B.T.; El-Sayed, Y.Y.; Blumenfeld, Y.J. Peripartum and Neonatal Outcomes of Small-For-Gestational-Age Infants with Gastroschisis. Prenat. Diagn. 2015, 35, 477–482. [Google Scholar] [CrossRef]
- Lausman, A.Y.; Langer, J.C.; Tai, M.; Seaward, G.R.; Windrim, R.C.; Kelly, E.N.; Ryan, G. Gastroschisis: What is the average gestational age of spontaneous delivery? J. Pediatr. Surg. 2007, 42, 1816–1821. [Google Scholar] [CrossRef]
- Overcash, R.T.; DeUgarte, D.A.; Stephenson, M.L.; Gutkin, R.M.; Norton, M.E.; Parmar, S.; Porto, M.; Poulain, F.R.; Schrimmer, D.B. Factors associated with gastroschisis outcomes. Obstet. Gynecol. 2014, 124, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barseghyan, K.; Aghajanian, P.; Miller, D.A. The prevalence of preterm births in pregnancies complicated with fetal gastroschisis. Arch. Gynecol. Obstet. 2012, 286, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.J.; Klein, N.; Chitty, L.S.; Kocjan, G.; Walshe, D.; Goulding, M.; Geary, M.P.; Pierro, A.; Rodeck, C.H. Intra-amniotic inflammation in human gastroschisis: Possible aetiology of postnatal bowel dysfunction. Br. J. Obstet. Gynaecol. 1998, 105, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Burc, L.; Volumenie, J.L.; de Lagausie, P.; Guibourdenche, J.; Oury, J.-F.; Vuillard, E.; Sibony, O.; Blot, P.; Saizou, C.; Luton, D. Amniotic fluid inflammatory proteins and digestive compounds profile in fetuses with gastroschisis undergoing amnioexchange. BJOG 2004, 111, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Gamba, P.; Midrio, P. Abdominal wall defects: Prenatal diagnosis, newborn management, and long-term outcomes. Semin. Pediatr. Surg. 2014, 23, 283–290. [Google Scholar] [CrossRef]
- South, A.P.; Stutey, K.M.; Meinzen-Derr, J. Metaanalysis of the prevalence of intrauterine fetal death in gastroschisis. Am. J. Obstet. Gynecol. 2013, 209, 114.e1–114.e13. [Google Scholar] [CrossRef]
- Sparks, T.N.; Shaffer, B.L.; Page, J.; Caughey, A.B. Gastroschisis: Mortality risks with each additional week of expectant management. Am. J. Obstet. Gynecol. 2017, 216, 66.e1–66.e7. [Google Scholar] [CrossRef]
- Landisch, R.M.; Yin, Z.; Christensen, M.; Szabo, A.; Wagner, A.J. Outcomes of gastroschisis early delivery: A systematic review and meta-analysis. J. Pediatr. Surg. 2017, 52, 1962–1971. [Google Scholar] [CrossRef]
- Shamshirsaz, A.A.; Lee, T.C.; Hair, A.B.; Erfani, H.; Espinoza, J.; Shamshirsaz, A.A.; Fox, K.A.; Gandhi, M.; Nassr, A.A.; Abrams, S.A.; et al. Elective delivery at 34 weeks vs routine obstetric care in fetal gastroschisis: Randomized controlled trial. Ultrasound Obstet. Gynecol. 2020, 55, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Logghe, H.L.; Mason, G.C.; Thornton, J.G.; Stringer, M.D. A randomized controlled trial of elective preterm delivery of fetuses with gastroschisis. J. Pediatr. Surg. 2005, 40, 1726–1731. [Google Scholar] [CrossRef]
- Grant, N.H.; Dorling, J.; Thornton, J.G. Elective preterm birth for fetal gastroschisis. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.M.; Goetzinger, K.R.; Biggio, J.R.; Macones, G.A. Timing of elective delivery in gastroschisis: A decision and cost-effectiveness analysis. Ultrasound Obstet. Gynecol. 2015, 46, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, A. Gastroschisis Outcomes of Delivery (GOOD) Study. ClinicalTrials.gov Identifier: NCT02774746. Available online: https://clinicaltrials.gov/ct2/show/NCT02774746?cond=Gastroschisis&draw=2&rank=3 (accessed on 4 August 2020).
- Puligandla, P.S.; Janvier, A.; Flageole, H.; Bouchard, S.; Laberge, J.M. Routine cesarean delivery does not improve the outcome of infants with gastroschisis. J. Pediatr. Surg. 2004, 39, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Emusu, D.; Aliyu, Z.Y.; Pierre-Louis, B.J.; Druschel, C.M.; Kirby, R.S. Mode of delivery and neonatal survival of infants with isolated gastroschisis. Obstet. Gynecol. 2004, 104, 678–683. [Google Scholar] [CrossRef]
- Boutros, J.; Regier, M.; Skarsgard, E.D.; Canadian Pediatric Surgery Network. Is timing everything? The influence of gestational age, birth weight, route, and intent of delivery on outcome in gastroschisis. J. Pediatr. Surg. 2009, 44, 912–917. [Google Scholar] [CrossRef]
- Lopez, A.; Benjamin, R.H.; Raut, J.R.; Ramakrishnan, A.; Mitchell, L.E.; Tsao, K.; Johnson, A.; Langlois, P.H.; Swartz, M.D.; Agopian, A.J. Mode of delivery and mortality among neonates with gastroschisis: A population-based cohort in Texas. Paediatr. Perinat. Epidemiol. 2019, 33, 204–212. [Google Scholar] [CrossRef]
- Segel, S.Y.; Marder, S.J.; Parry, S.; Macones, G.A. Fetal abdominal wall defects and mode of delivery: A systematic review. Obstet. Gynecol. 2001, 98 Pt 1, 867–873. [Google Scholar] [CrossRef]
- Kirollos, D.W.; Abdel-Latif, M.E. Mode of delivery and outcomes of infants with gastroschisis: A meta-analysis of observational studies. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F355–F363. [Google Scholar] [CrossRef]
- Girsen, A.I.; Wallenstein, M.B.; Davis, A.S.; Hintz, S.R.; Desai, A.K.; Mansour, T.; Merritt, T.A.; Druzin, M.L.; Oshiro, B.T.; Blumenfeld, Y.J. Effect of antepartum meconium staining on perinatal and neonatal outcomes among pregnancies with gastroschisis. J. Matern. Fetal Neonatal Med. 2016, 29, 2500–2504. [Google Scholar] [CrossRef]
- Jansen, L.A.; Safavi, A.; Lin, Y.; MacNab, Y.C.; Skarsgard, E.D.; Canadian Pediatric Surgery Network (CAPSNet). Preclosure fluid resuscitation influences outcome in gastroschisis. Am. J. Perinatol. 2012, 29, 307–312. [Google Scholar] [CrossRef]
- Ramadan, G.; Rex, D.; Okoye, B.; Kennea, N.L. Early high C-reactive protein in infants with open abdominal wall defects does not predict sepsis or adverse outcome. Acta Paediatr. 2010, 99, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; Leonard, M.; Hall, E.S.; Perez, J.; Wessel, J.; Kingma, P.S. Evaluation of Early Onset Sepsis, Complete Blood Count, and Antibiotic Use in Gastroschisis. Am. J. Perinatol. 2018, 35, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Yaster, M.; Scherer, T.L.; Stone, M.M.; Schleien, C.L.; Wetzel, R.C.; Buck, J.R.; Nichols, D.; Colombani, P.M.; Dudgeon, D.L. Prediction of successful primary closure of congenital abdominal wall defects using intraoperative measurements. J. Pediatr. Surg. 1989, 24, 1217–1220. [Google Scholar] [CrossRef]
- Olesevich, M.; Alexander, F.; Khan, M.; Cotman, K. Gastroschisis revisited: Role of intraoperative measurement of abdominal pressure. J. Pediatr. Surg. 2005, 40, 789–792. [Google Scholar] [CrossRef]
- Petrosyan, M.; Sandler, A.D. Closure methods in gastroschisis. Semin. Pediatr. Surg. 2018, 27, 304–308. [Google Scholar] [CrossRef]
- Sandler, A.; Lawrence, J.; Meehan, J.; Phearman, L.; Soper, R. A “plastic” sutureless abdominal wall closure in gastroschisis. J. Pediatr. Surg. 2004, 39, 738–741. [Google Scholar] [CrossRef]
- Kidd, J.N., Jr.; Jackson, R.J.; Smith, S.D.; Wagner, C.W. Evolution of staged versus primary closure of gastroschisis. Ann. Surg. 2003, 237, 759–765. [Google Scholar] [CrossRef]
- Schlatter, M.; Norris, K.; Uitvlugt, N.; DeCou, J.; Connors, R. Improved outcomes in the treatment of gastroschisis using a preformed silo and delayed repair approach. J. Pediatr. Surg. 2003, 38, 459–464. [Google Scholar] [CrossRef]
- Kunz, S.N.; Tieder, J.S.; Whitlock, K.; Jackson, J.C.; Avansino, J.R. Primary fascial closure versus staged closure with silo in patients with gastroschisis: A meta-analysis. J. Pediatr. Surg. 2013, 48, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, R.B.; Raymond, S.L.; St Peter, S.D.; Downard, C.D.; Qureshi, F.G.; Renaud, E.; Danielson, P.D.; Islam, S. Immediate Versus Silo Closure for Gastroschisis: Results of a Large Multicenter Study. J. Pediatr. Surg. 2020, 55, 1280–1285. [Google Scholar] [CrossRef]
- Pastor, A.C.; Phillips, J.D.; Fenton, S.J.; Meyers, R.L.; Lamm, A.W.; Raval, M.V.; Lehman, E.; Karp, T.B.; Wales, P.W.; Langer, J.C. Routine use of a SILASTIC spring-loaded silo for infants with gastroschisis: A multicenter randomized controlled trial. J. Pediatr. Surg. 2008, 43, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Poola, A.S.; Aguayo, P.; Fraser, J.D.; Hendrickson, R.J.; Weaver, K.L.; Gonzalez, K.W.; Peter, S.D.S. Primary Closure versus Bedside Silo and Delayed Closure for Gastroschisis: A Truncated Prospective Randomized Trial. Eur. J. Pediatr. Surg. 2019, 29, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Emami, C.N.; Youssef, F.; Baird, R.J.; Laberge, J.-M.; Skarsgard, E.D.; Puligandla, P.S. A risk-stratified comparison of fascial versus flap closure techniques on the early outcomes of infants with gastroschisis. J. Pediatr. Surg. 2015, 50, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Pet, G.E.; Stark, R.A.; Meehan, J.J.; Javid, P.J. Outcomes of bedside sutureless umbilical closure without endotracheal intubation for gastroschisis repair in surgical infants. Am. J. Surg. 2017, 213, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Witt, R.G.; Zobel, M.; Padilla, B.; Lee, H.; MacKenzie, T.C.; Vu, L. Evaluation of Clinical Outcomes of Sutureless vs Sutured Closure Techniques in Gastroschisis Repair. JAMA Surg. 2019, 154, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzoni, M.; Jaramillo, J.D.; Dunlap, J.L.; Abrajano, C.; Stack, S.W.; Hintz, S.R.; Hernandez-Boussard, T.; Dutta, S. Sutureless vs Sutured Gastroschisis Closure: A Prospective Randomized Controlled Trial. J. Am. Coll. Surg. 2017, 224, 1091–1096.e1. [Google Scholar] [CrossRef]
- Youssef, F.; Gorgy, A.; Arbash, G.; Puligandla, P.S.; Baird, R.J. Flap versus fascial closure for gastroschisis: A systematic review and meta-analysis. J. Pediatr. Surg. 2016, 51, 718–725. [Google Scholar] [CrossRef]
- Jadcherla, S.R.; Gupta, A.; Stoner, E.; Fernandez, S.; Caniano, D.; Rudolph, C.D. Neuromotor markers of esophageal motility in feeding intolerant infants with gastroschisis. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 158–164. [Google Scholar] [CrossRef]
- Jayanthi, S.; Seymour, P.; Puntis, J.W.; Stringer, M.D. Necrotizing enterocolitis after gastroschisis repair: A preventable complication? J. Pediatr. Surg. 1998, 33, 705–707. [Google Scholar] [CrossRef]
- Curry, J.I.; Lander, A.D.; Stringer, M.D.; BAPS Multicentre Research Committee. A multicenter, randomized, double-blind, placebo-controlled trial of the prokinetic agent erythromycin in the postoperative recovery of infants with gastroschisis. J. Pediatr. Surg. 2004, 39, 565–569. [Google Scholar] [CrossRef]
- Lusk, L.A.; Brown, E.G.; Overcash, R.T.; Grogan, T.R.; Keller, R.L.; Kim, J.H.; Poulain, F.R.; Shew, S.B.; Uy, C.; DeUgarte, D.A. University of California Fetal Consortium. Multi-institutional practice patterns and outcomes in uncomplicated gastroschisis: A report from the University of California Fetal Consortium (UCfC). J. Pediatr. Surg. 2014, 49, 1782–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeUgarte, D.A.; Calkins, K.L.; Guner, Y.; Kim, J.; Kling, K.; Kramer, K.; Lee, H.; Lusk, L.; Saadai, P.; Uy, C.; et al. University of California Fetal Consortium. Adherence to and outcomes of a University-Consortium gastroschisis pathway. J. Pediatr. Surg. 2020, 55, 45–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fullerton, B.S.; Velazco, C.S.; Sparks, E.A.; Morrow, K.A.; Edwards, E.M.; Soll, R.F.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Contemporary Outcomes of Infants with Gastroschisis in North America: A Multicenter Cohort Study. J. Pediatr. 2017, 188, 192–197.e6. [Google Scholar] [CrossRef] [PubMed]
- Fawley, J.A.; Abdelhafeez, A.H.; Schultz, J.A.; Ertl, A.; Cassidy, L.D.; St Peter, S.; Wagner, A.J. The risk of midgut volvulus in patients with abdominal wall defects: A multi-institutional study. J. Pediatr. Surg. 2017, 52, 26–29. [Google Scholar] [CrossRef] [PubMed]
- De Marco, G.; Sordino, D.; Bruzzese, E.; Di Caro, S.; Mambretti, D.; Tramontano, A.; Colombo, C.; Simoni, P.; Guarino, A. Early treatment with ursodeoxycholic acid for cholestasis in children on parenteral nutrition because of primary intestinal failure. Aliment. Pharmacol. Ther. 2006, 24, 387–394. [Google Scholar] [CrossRef]
- Muhammed, R.; Bremner, R.; Protheroe, S.; Johnson, T.; Holden, C.; Murphy, M.S. Resolution of parenteral nutrition-associated jaundice on changing from a soybean oil emulsion to a complex mixed-lipid emulsion. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 797–802. [Google Scholar] [CrossRef]
- Jensen, A.R.; Goldin, A.B.; Koopmeiners, J.S.; Stevens, J.; Waldhausen, J.H.; Kim, S.S. The association of cyclic parenteral nutrition and decreased incidence of cholestatic liver disease in patients with gastroschisis. J. Pediatr. Surg. 2009, 44, 183–189. [Google Scholar] [CrossRef]
- Amin, S.C.; Pappas, C.; Iyengar, H.; Maheshwari, A. Short bowel syndrome in the NICU. Clin. Perinatol. 2013, 40, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Iyer, K.R. Surgical management of short bowel syndrome. JPEN J. Parenter. Enteral Nutr. 2014, 38 (Suppl. l), 53S–59S. [Google Scholar] [CrossRef]
- Yardley, I.E.; Bostock, E.; Jones, M.O.; Turnock, R.R.; Corbett, H.J.; Losty, P.D. Congenital abdominal wall defects and testicular maldescent—A 10-year single-center experience. J. Pediatr. Surg. 2012, 47, 1118–1122. [Google Scholar] [CrossRef]
- Hill, S.J.; Durham, M.M. Management of cryptorchidism and gastroschisis. J. Pediatr. Surg. 2011, 46, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.; Cheong, L.H.; Emil, S.; Canadian Pediatric Surgery Network (CAPSNet). Gastroschisis outcomes in North America: A comparison of Canada and the United States. J. Pediatr. Surg. 2016, 51, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Friedmacher, F.; Hock, A.; Castellani, C.; Avian, A.; Höllwarth, M.E. Gastroschisis-related complications requiring further surgical interventions. Pediatr. Surg. Int. 2014, 30, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Emil, S.; Canvasser, N.; Chen, T.; Friedrich, E.; Su, W. Contemporary 2-year outcomes of complex gastroschisis. J. Pediatr. Surg. 2012, 47, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Cowan, K.N.; Puligandla, P.S.; Laberge, J.M.; Skarsgard, E.D.; Bouchard, S.; Yanchar, N.; Kim, P.; Lee, S.; McMillan, D.; von Dadelszen, P.; et al. The gastroschisis prognostic score: Reliable outcome prediction in gastroschisis. J. Pediatr. Surg. 2012, 47, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Puligandla, P.S.; Baird, R.; Skarsgard, E.D.; Emil, S.; Laberge, J.M.; Canadian Pediatric Surgery Network (CAPSNet). Outcome prediction in gastroschisis—The gastroschisis prognostic score (GPS) revisited. J. Pediatr. Surg. 2017, 52, 718–721. [Google Scholar] [CrossRef]
- Kokesova, A.; Coufal, S.; Frybova, B.; Kverka, M.; Rygl, M. The intestinal fatty acid-binding protein as a marker for intestinal damage in gastroschisis. PLoS ONE 2019, 14, e0210797. [Google Scholar] [CrossRef]
- Clausen, N.G.; Hansen, T.G.; Disma, N. Anesthesia Neurotoxicity in the Developing Brain: Basic Studies Relevant for Neonatal or Perinatal Medicine. Clin. Perinatol. 2019, 46, 647–656. [Google Scholar] [CrossRef]
- Andropoulos, D.B. Effect of Anesthesia on the Developing Brain: Infant and Fetus. Fetal Diagn. Ther. 2018, 43, 1–11. [Google Scholar] [CrossRef]
- Minutillo, C.; Rao, S.C.; Pirie, S.; McMichael, J.; Dickinson, J.E. Growth and developmental outcomes of infants with gastroschisis at one year of age: A retrospective study. J. Pediatr. Surg. 2013, 48, 1688–1696. [Google Scholar] [CrossRef]
- South, A.P.; Marshall, D.D.; Bose, C.L.; Laughon, M.M. Growth and neurodevelopment at 16 to 24 months of age for infants born with gastroschisis. J. Perinatol. 2008, 28, 702–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Manen, M.; Hendson, L.; Wiley, M.; Evans, M.; Taghaddos, S.; Dinu, I. Early childhood outcomes of infants born with gastroschisis. J. Pediatr. Surg. 2013, 48, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.L.; Minutillo, C.; Hart, S.; Warner, T.M.; Ravikumara, M.; Nathan, E.A.; Dickinson, J.E. The long term physical consequences of gastroschisis. J. Pediatr. Surg. 2014, 49, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.W.; Stringer, M.D. The survivors of gastroschisis. Arch. Dis. Child. 1997, 77, 158–160. [Google Scholar] [CrossRef]
- Gorra, A.S.; Needelman, H.; Azarow, K.S.; Roberts, H.J.; Jackson, B.J.; Cusick, R.A. Long-term neurodevelopmental outcomes in children born with gastroschisis: The tiebreaker. J. Pediatr. Surg. 2012, 47, 125–129. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, A.; Walker, K.; Holland, A.J.A. Neurodevelopmental outcome of infants with gastroschisis at one-year follow-up. J. Neonatal. Surg. 2015, 4, 12. [Google Scholar]
- Harris, E.L.; Hart, S.J.; Minutillo, C.; Ravikumara, M.; Warner, T.M.; Williams, Y.; Nathan, E.A.; Dickinson, J.E. The long-term neurodevelopmental and psychological outcomes of gastroschisis: A cohort study. J. Pediatr. Surg. 2016, 51, 549–553. [Google Scholar] [CrossRef]
- Lap, C.C.; Bolhuis, S.W.; Van Braeckel, K.N.; Reijneveld, S.A.; Manten, G.T.R.; Bos, A.F.; Hulscher, J.B.F. Functional outcome at school age of children born with gastroschisis. Early Hum. Dev. 2017, 106–107, 47–52. [Google Scholar] [CrossRef]
- Tosello, B.; Zahed, M.; Guimond, F.; Baumstarck, K.; Faure, A.; Michel, F.; Claris, O.; Massardier, J.; Gire, C.; Merrot, T. Neurodevelopment and Health-Related Quality of Life in Infants Born with Gastroschisis: A 6-Year Retrospective French Study. Eur. J. Pediatr. Surg. 2017, 27, 352–360. [Google Scholar]
- Carpenter, J.L.; Wiebe, T.L.; Cass, D.L.; Olutoye, O.O.; Lee, T.C. Assessing quality of life in pediatric gastroschisis patients using the Pediatric Quality of Life Inventory survey: An institutional study. J. Pediatr. Surg. 2016, 51, 726–729. [Google Scholar] [CrossRef]
- Arnold, H.E.; Baxter, K.J.; Short, H.L.; Travers, C.; Bhatia, A.; Durham, M.M.; Raval, M.V. Short-term and family-reported long-term outcomes of simple versus complicated gastroschisis. J. Surg. Res. 2018, 224, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Snoep, M.C.; de Heus, R.; Manten, G.T.R.; Lap, C.C.M.M.; Snoeker, B.A.M.; Lindeboom, M.Y.A. Gastro-intestinal function and quality of life are favorable in adolescent and adult gastroschisis patients. Early Hum. Dev. 2020, 141, 104936. [Google Scholar] [CrossRef] [PubMed]
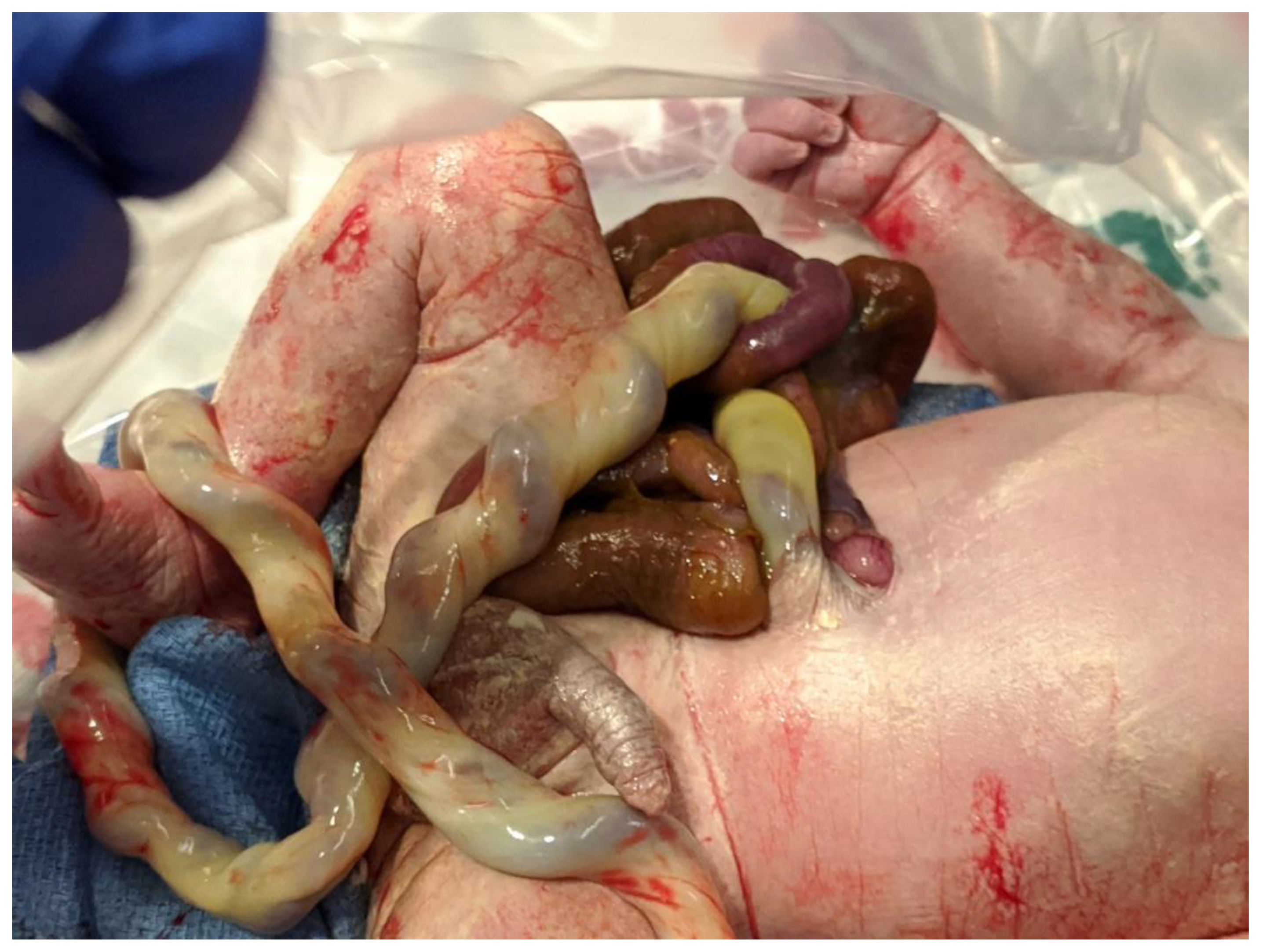
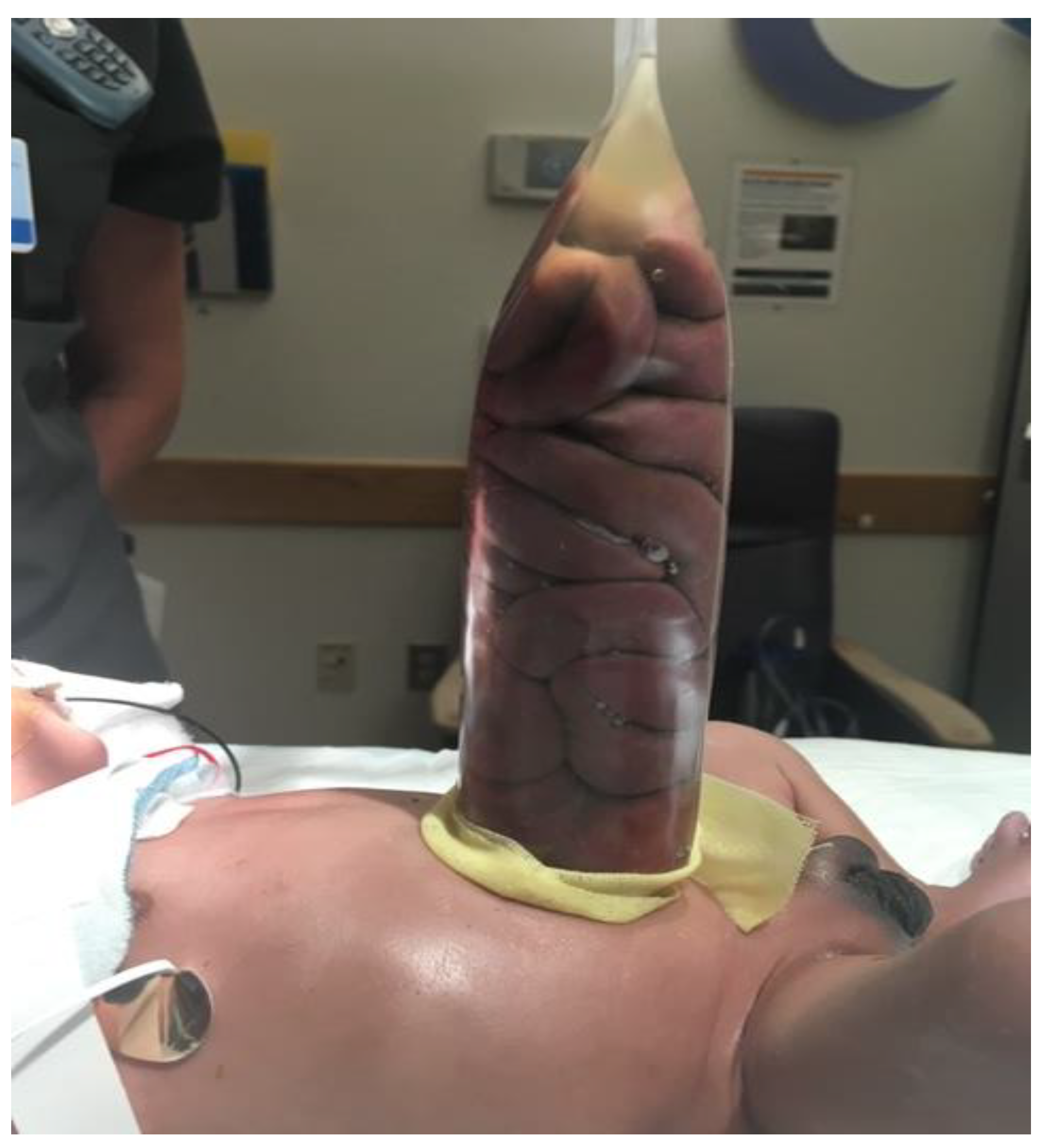
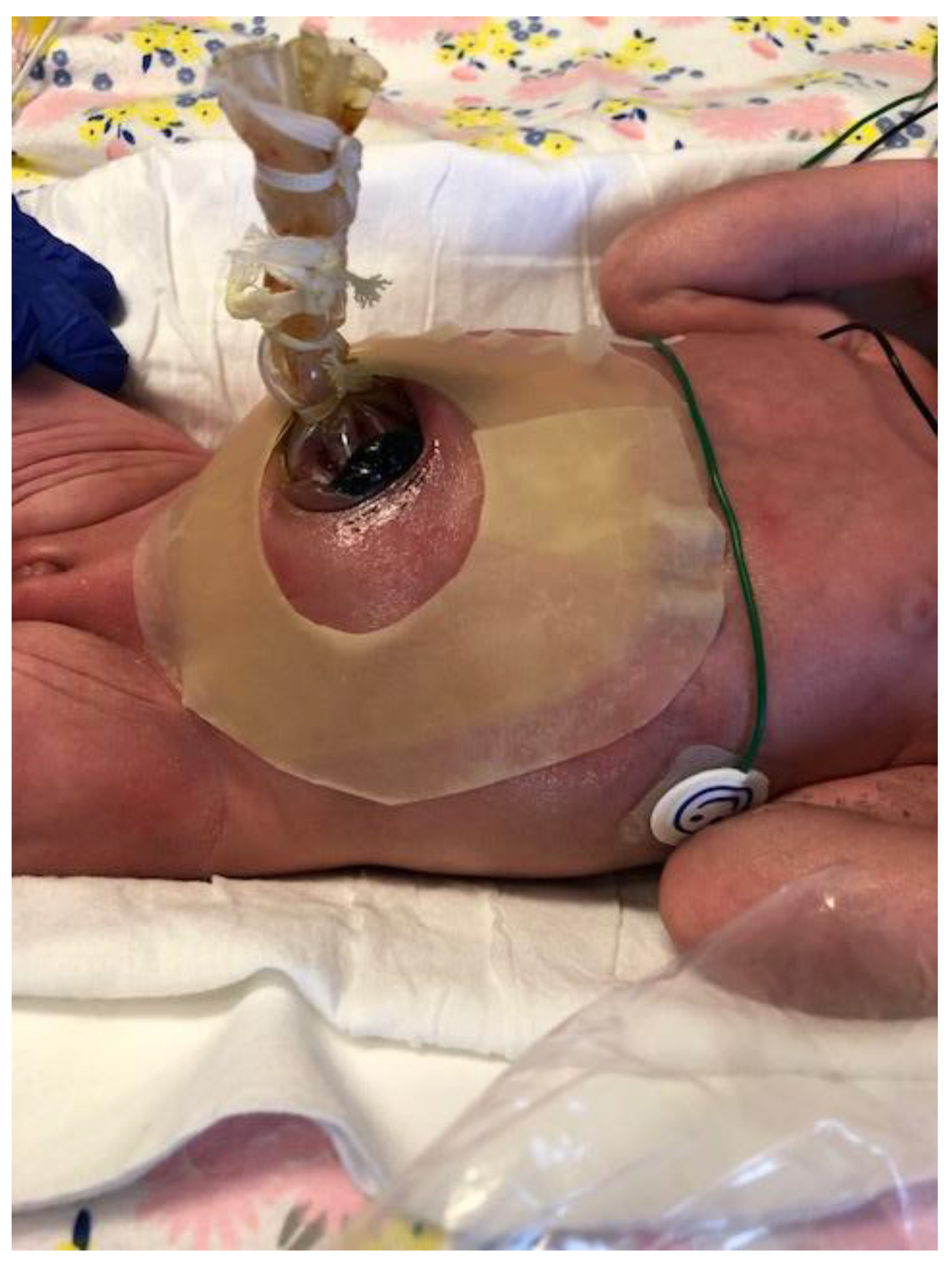

| Parameter | Description | Score | Description | Score | Description | Score |
|---|---|---|---|---|---|---|
| Matting | None | 0 | Mild | 1 | Severe | 4 |
| Atresia | None | 0 | Suspected | 1 | Present | 2 |
| Perforation | None | 0 | Present | 2 | ||
| Necrosis | None | 0 | Present | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhat, V.; Moront, M.; Bhandari, V. Gastroschisis: A State-of-the-Art Review. Children 2020, 7, 302. https://0-doi-org.brum.beds.ac.uk/10.3390/children7120302
Bhat V, Moront M, Bhandari V. Gastroschisis: A State-of-the-Art Review. Children. 2020; 7(12):302. https://0-doi-org.brum.beds.ac.uk/10.3390/children7120302
Chicago/Turabian StyleBhat, Vishwanath, Matthew Moront, and Vineet Bhandari. 2020. "Gastroschisis: A State-of-the-Art Review" Children 7, no. 12: 302. https://0-doi-org.brum.beds.ac.uk/10.3390/children7120302




