The Current Status of Neuroprotection in Congenital Heart Disease
Abstract
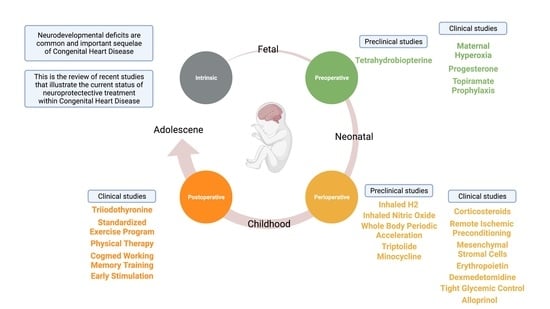
:1. Introduction
1.1. Boston Circulatory Arrest Trial
1.2. Single Ventricle Reconstruction Trial
1.3. Risk Factors Associated with Neurological Deficits in Children with CHD
2. Maternal Neuroprotection—Clinical Studies
2.1. Maternal Hyperoxia
2.2. Progesterone
2.3. Topiramate Prophylaxis
3. Maternal Neuroprotection—Preclinical Studies for Future Translation
Tetrahydrobiopterin
4. Peri-Operative Neuroprotection—Clinical Studies
4.1. Corticosteroids
4.2. Remote Ischemic Preconditioning
4.3. Mesenchymal Stromal Cells
4.4. Erythropoietin
4.5. Dexmedetomidine
4.6. Tight Glycemic Control
4.7. Allopurinol
5. Peri-Operative Neuroprotection—Preclinical Studies for Future Translation
5.1. Inhaled H2
5.2. Inhaled Nitric Oxide
5.3. Whole Body Periodic Acceleration
5.4. Triptolide
5.5. Minocycline
6. Post-Operative Neuroprotection—Clinical Studies
6.1. Triiodothyronine
6.2. Standardized Exercise Program
6.3. Physical Therapy
6.4. Cogmed Working Memory Training
6.5. Early Stimulation
7. Socioeconomic Status
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilboa, S.M.; Salemi, J.; Nembhard, W.N.; Fixler, D.E.; Correa, A. Mortality Resulting from Congenital Heart Disease among Children and Adults in the United States, 1999 to 2006. Circulation 2010, 122, 2254–2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, P.-L.; Stefanescu, A.; Samoukovic, G.; Tchervenkov, C.I. The Challenge of Congenital Heart Disease Worldwide: Epidemiologic and Demographic Facts. Semin. Thorac. Cardiovasc. Surgery Pediatr. Card. Surg. Annu. 2010, 13, 26–34. [Google Scholar] [CrossRef]
- Marelli, A.J.; Ionescu-Ittu, R.; Mackie, A.; Guo, L.; Dendukuri, N.; Kaouache, M. Lifetime Prevalence of Congenital Heart Disease in the General Population from 2000 to 2010. Circulation 2014, 130, 749–756. [Google Scholar] [CrossRef] [Green Version]
- Boneva, R.S.; Botto, L.D.; Moore, C.A.; Yang, Q.; Correa, A.; Erickson, J.D. Mortality Associated with Congenital Heart Defects in the United States. Circulation 2001, 103, 2376–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, P.D.; Ishibashi, N.; Jonas, R.A. Neurodevelopmental Abnormalities and Congenital Heart Disease. Circ. Res. 2017, 120, 960–977. [Google Scholar] [CrossRef] [Green Version]
- Marino, B.S.; Lipkin, P.; Newburger, J.W.; Peacock, G.; Gerdes, M.; Gaynor, J.W.; Mussatto, K.A.; Uzark, K.; Goldberg, C.S.; Johnsonjr, W.H.; et al. Neurodevelopmental Outcomes in Children with Congenital Heart Disease: Evaluation and Management. Circulation 2012, 126, 1143–1172. [Google Scholar] [CrossRef] [Green Version]
- Moons, P.; Bovijn, L.; Budts, W.; Belmans, A.; Gewillig, M. Temporal Trends in Survival to Adulthood among Patients Born with Congenital Heart Disease from 1970 to 1992 in Belgium. Circulation 2010, 122, 2264–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newburger, J.W.; Sleeper, L.A.; Bellinger, D.C.; Goldberg, C.S.; Tabbutt, S.; Lu, M.; Mussatto, K.A.; Williams, I.A.; Gustafson, K.E.; Mital, S.; et al. Early Developmental Outcome in Children with Hypoplastic Left Heart Syndrome and Related Anomalies. Circulation 2012, 125, 2081–2091. [Google Scholar] [CrossRef] [Green Version]
- Calderon, J.; Bonnet, D.; Courtin, C.; Concordet, S.; Plumet, M.-H.; Angeard, N. Executive function and theory of mind in school-aged children after neonatal corrective cardiac surgery for transposition of the great arteries. Dev. Med. Child Neurol. 2010, 52, 1139–1144. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Wypij, D.; Rivkin, M.J.; DeMaso, D.R.; Robertson, R.; Dunbar-Masterson, C.; Rappaport, L.A.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Adolescents with d-Transposition of the Great Arteries Corrected with the Arterial Switch Procedure. Circulation 2011, 124, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Mahle, W.T.; Wernovsky, G. Long-Term Developmental Outcome Of Children with Complex Congenital Heart Disease. Clin. Perinatol. 2001, 28, 235–247. [Google Scholar] [CrossRef]
- Gaynor, J.W.; Nord, A.S.; Wernovsky, G.; Bernbaum, J.; Solot, C.B.; Burnham, N.; Zackai, E.; Heagerty, P.J.; Clancy, R.R.; Nicolson, S.C.; et al. Apolipoprotein E Genotype Modifies the Risk of Behavior Problems after Infant Cardiac Surgery. Pediatrics 2009, 124, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, D.C.; Newburger, J.W.; Wypij, D.; Kuban, K.C.K.; Duplesssis, A.J.; Rappaport, L.A. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol. Young 2009, 19, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellinger, D.C.; Wypij, D.; Duplessis, A.J.; Rappaport, L.A.; Jonas, R.A.; Wernovsky, G.; Newburger, J.W. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: The Boston Circulatory Arrest Trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1385–1396. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, D.C.; Wypij, D.; Kuban, K.; Rappaport, L.A.; Hickey, P.R.; Wernovsky, G.; Jonas, R.A.; Newburger, J.W. Developmental and Neurological Status of Children at 4 Years of Age after Heart Surgery with Hypothermic Circulatory Arrest or Low-Flow Cardiopulmonary Bypass. Circulation 1999, 100, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Jonas, R.A.; Wernovsky, G.; Wypij, D.; Hickey, P.R.; Kuban, K.; Farrell, D.M.; Holmes, G.L.; Helmers, S.L.; Constantinou, J.; et al. A Comparison of the Perioperative Neurologic Effects of Hypothermic Circulatory Arrest versus Low-Flow Cardiopulmonary Bypass in Infant Heart Surgery. N. Engl. J. Med. 1993, 329, 1057–1064. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Rappaport, L.A.; Wypij, D.; Wernovsky, G.; Newburger, J.W. Patterns of Developmental Dysfunction after Surgery during Infancy to Correct Transposition of the Great Arteries. J. Dev. Behav. Pediatr. 1997, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Robson, V.K.; Stopp, C.; Wypij, D.; Dunbar-Masterson, C.; Bellinger, D.C.; DeMaso, D.R.; Rappaport, L.A.; Newburger, J.W. Longitudinal Associations between Neurodevelopment and Psychosocial Health Status in Patients with Repaired D-Transposition of the Great Arteries. J. Pediatr. 2019, 204, 38–45.e1. [Google Scholar] [CrossRef] [PubMed]
- DeMaso, D.R.; Calderon, J.; Taylor, G.A.; Holland, J.E.; Stopp, C.; White, M.T.; Bellinger, D.C.; Rivkin, M.J.; Wypij, D.; Newburger, J.W. Psychiatric Disorders in Adolescents with Single Ventricle Congenital Heart Disease. Pediatrics 2017, 139, e20162241. [Google Scholar] [CrossRef] [Green Version]
- Bellinger, D.C.; Watson, C.G.; Rivkin, M.J.; Robertson, R.L.; Roberts, A.E.; Stopp, C.; Dunbar-Masterson, C.; Bernson, D.; DeMaso, D.R.; Wypij, D.; et al. Neuropsychological Status and Structural Brain Imaging in Adolescents with Single Ventricle Who Underwent the Fontan Procedure. J. Am. Heart Assoc. 2015, 4, e002302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sananes, R.; Goldberg, C.S.; Newburger, J.W.; Hu, C.; Trachtenberg, F.; Gaynor, J.W.; Mahle, W.T.; Miller, T.; Uzark, K.; Mussatto, K.A.; et al. Six-Year Neurodevelopmental Outcomes for Children with Single-Ventricle Physiology. Pediatrics 2021, 147, e2020014589. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Hu, C.; Brosig, C.; Gaynor, J.W.; Mahle, W.T.; Miller, T.; Mussatto, K.A.; Sananes, R.; Uzark, K.; Trachtenberg, F.; et al. Behavior and Quality of Life at 6 Years for Children with Hypoplastic Left Heart Syndrome. Pediatrics 2019, 144, e20191010. [Google Scholar] [CrossRef]
- Sanz, J.H.; Berl, M.; Ma, A.C.A.; Wang, J.; Cheng, Y.I.; Donofrio, M.T. Prevalence and pattern of executive dysfunction in school age children with congenital heart disease. Congenit. Heart Dis. 2017, 12, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Holm, I.; Fredriksen, P.M.; Fosdahl, M.A.; Olstad, M.; Vøllestad, N. Impaired Motor Competence in School-aged Children with Complex Congenital Heart Disease. Arch. Pediatr. Adolesc. Med. 2007, 161, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Limperopoulos, C.; Majnemer, A.; Shevell, M.I.; Rosenblatt, B.; Rohlicek, C.; Tchervenkov, C.; Darwish, H. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics 2001, 108, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, S.L.; Carey, H.; Chisolm, J.L.; Heathcock, J.C.; Steward, D. Early Results of Neurodevelopment Following Hybrid Stage I for Hypoplastic Left Heart Syndrome. Pediatr. Cardiol. 2014, 36, 685–691. [Google Scholar] [CrossRef]
- Cassidy, A.R.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C. Executive Function in Children and Adolescents with Critical Cyanotic Congenital Heart Disease. J. Int. Neuropsychol. Soc. 2015, 21, 34–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunbar-Masterson, C.; Wypij, D.; Bellinger, D.C.; Rappaport, L.A.; Baker, A.L.; Jonas, R.A.; Newburger, J.W. General Health Status of Children with d-Transposition of the Great Arteries after the Arterial Switch Operation. Circulation 2001, 104, I-138. [Google Scholar] [CrossRef]
- Hövels-Gürich, H.H.; Konrad, K.; Skorzenski, D.; Herpertz-Dahlmann, B.; Messmer, B.J.; Seghaye, M.-C. Attentional Dysfunction in Children after Corrective Cardiac Surgery in Infancy. Ann. Thorac. Surg. 2007, 83, 1425–1430. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Harrison, T.; Heathcock, J. Infants with complex congenital heart diseases show poor short-term memory in the mobile paradigm at 3 months of age. Infant Behav. Dev. 2015, 40, 12–19. [Google Scholar] [CrossRef]
- Homsy, J.; Zaidi, S.; Shen, Y.; Ware, J.S.; Samocha, K.E.; Karczewski, K.J.; DePalma, S.R.; McKean, D.; Wakimoto, H.; Gorham, J.; et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015, 350, 1262–1266. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.C.; Homsy, J.; Zaidi, S.; Lu, Q.; Morton, S.; DePalma, S.R.; Zeng, X.; Qi, H.; Chang, W.; Sierant, M.C.; et al. Contribution of rare inherited and de novo variants in 2871 congenital heart disease probands. Nat. Genet. 2017, 49, 1593–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licht, D.J.; Wang, D.; Silvestre, D.W.; Nicolson, S.C.; Montenegro, L.M.; Wernovsky, G.; Tabbutt, S.; Durning, S.M.; Shera, D.M.; Gaynor, J.W.; et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J. Thorac. Cardiovasc. Surg. 2004, 128, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, G.M.; Brosig, C.L.; Mussatto, K.A.; Tweddell, J.S.; Ghanayem, N.S. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. J. Thorac. Cardiovasc. Surg. 2013, 146, 1153–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Macgowan, C.; Sled, J.G.; Yoo, S.-J.; Manlhiot, C.; Porayette, P.; Grosse-Wortmann, L.; Jaeggi, E.; McCrindle, B.W.; Kingdom, J.; et al. Reduced Fetal Cerebral Oxygen Consumption Is Associated with Smaller Brain Size in Fetuses with Congenital Heart Disease. Circulation 2015, 131, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- Moller, J.H.; Taubert, K.A.; Allen, H.D.; Clark, E.B.; Lauer, R.M. Cardiovascular health and disease in children: Current status. A Special Writing Group from the Task Force on Children and Youth, American Heart Association. Circulation 1994, 89, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Andropoulos, D.B.; Hunter, J.V.; Nelson, D.P.; Stayer, S.A.; Stark, A.R.; McKenzie, E.D.; Heinle, J.S.; Graves, D.; Fraser, C.D. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J. Thorac. Cardiovasc. Surg. 2010, 139, 543–556. [Google Scholar] [CrossRef] [Green Version]
- Beca, J.; Gunn, J.K.; Coleman, L.; Hope, A.; Reed, P.W.; Hunt, R.W.; Finucane, K.; Brizard, C.; Dance, B.; Shekerdemian, L.S. New White Matter Brain Injury after Infant Heart Surgery Is Associated with Diagnostic Group and the Use of Circulatory Arrest. Circulation 2013, 127, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Fudulu, D.; Angelini, G. Oxidative Stress after Surgery on the Immature Heart. Oxidative Med. Cell. Longev. 2016, 2016, 1971452. [Google Scholar] [CrossRef] [Green Version]
- Korotcova, L.; Kumar, S.; Agematsu, K.; Morton, P.D.; Jonas, R.A.; Ishibashi, N. Prolonged White Matter Inflammation after Cardiopulmonary Bypass and Circulatory Arrest in a Juvenile Porcine Model. Ann. Thorac. Surg. 2015, 100, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, E.Z.; Pertsovskaya, V.; Forbes, T.A.; Dupree, J.L.; Gallo, V. Prolonged Environmental Enrichment Promotes Developmental Myelination. Front. Cell Dev. Biol. 2021, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Adams-Chapman, I.; Stoll, B.J. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr. Opin. Infect. Dis. 2006, 19, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.; Farah, M.J.; Meaney, M.J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010, 11, 651–659. [Google Scholar] [CrossRef]
- Benavente-Fernandez, I.; Siddiqi, A.; Miller, S.P. Socioeconomic status and brain injury in children born preterm: Modifying neurodevelopmental outcome. Pediatr. Res. 2019, 87, 391–398. [Google Scholar] [CrossRef]
- Lawrence, K.M.; McGovern, P.E.; Mejaddam, A.; Rossidis, A.C.; Baumgarten, H.; Kim, A.; Grinspan, J.B.; Licht, D.; Didier, R.A.; Vossough, A.; et al. Chronic intrauterine hypoxia alters neurodevelopment in fetal sheep. J. Thorac. Cardiovasc. Surg. 2019, 157, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Andescavage, N.N.; Kapse, K.; Donofrio, M.T.; Jacobs, M.; Limperopoulos, C. Hemodynamic Responses of the Placenta and Brain to Maternal Hyperoxia in Fetuses with Congenital Heart Disease by Using Blood Oxygen–Level Dependent MRI. Radiology 2020, 294, 141–148. [Google Scholar] [CrossRef]
- Nicolaides, K.; Bradley, R.; Soothill, P.; Campbell, S.; Bilardo, C.; Gibb, D. Maternal Oxygen Therapy for Intrauterine Growth Retardation. Lancet 1987, 329, 942–945. [Google Scholar] [CrossRef]
- Liu, G.; Yan, Y.; Shi, B.; Huang, J.; Mu, H.; Li, C.; Chen, H.; Zhu, Z. Benefits of progesterone on brain immaturity and white matter injury induced by chronic hypoxia in neonatal rats. J. Thorac. Cardiovasc. Surg. 2020, 160, e55–e66. [Google Scholar] [CrossRef]
- Hirst, J.J.; Yawno, T.; Nguyen, P.; Walker, D.W. Stress in Pregnancy Activates Neurosteroid Production in the Fetal Brain. Neuroendocrinology 2006, 84, 264–274. [Google Scholar] [CrossRef]
- Yawno, T.; Yan, E.; Walker, D.; Hirst, J. Inhibition of neurosteroid synthesis increases asphyxia-induced brain injury in the late gestation fetal sheep. Neuroscience 2007, 146, 1726–1733. [Google Scholar] [CrossRef]
- Nguyen, P.N.; Billiards, S.S.; Walker, D.W.; Hirst, J.J. Changes in 5α-Pregnane Steroids and Neurosteroidogenic Enzyme Expression in Fetal Sheep with Umbilicoplacental Embolization. Pediatr. Res. 2003, 54, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, M.; Liere, P.; Ghoumari, A. Progesterone and fetal-neonatal neuroprotection. Best Pr. Res. Clin. Obstet. Gynaecol. 2020, 69, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Filippi, L.; Gerace, E.; Catarzi, S.; Guerrini, R.; Pellegrini-Giampietro, D.E. Neuroprotective effects of topiramate and memantine in combination with hypothermia in hypoxic-ischemic brain injury in vitro and in vivo. Neurosci. Lett. 2018, 668, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shuaib, A.; Li, Q.; Siddiqui, M.M. Neuroprotection by delayed administration of topiramate in a rat model of middle cerebral artery embolization. Brain Res. 1998, 804, 169–176. [Google Scholar] [CrossRef]
- Follett, P.L.; Deng, W.; Dai, W.; Talos, D.M.; Massillon, L.J.; Rosenberg, P.A.; Volpe, J.J.; Jensen, F.E. Glutamate Receptor-Mediated Oligodendrocyte Toxicity in Periventricular Leukomalacia: A Protective Role for Topiramate. J. Neurosci. 2004, 24, 4412–4420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoover, R.C.; Motta, M.; Davis, J.; Saatman, K.E.; Fujimoto, S.T.; Thompson, H.J.; Stover, J.F.; Dichter, M.A.; Twyman, R.; White, H.S.; et al. Differential Effects of the Anticonvulsant Topiramate on Neurobehavioral and Histological Outcomes following Traumatic Brain Injury in Rats. J. Neurotrauma 2004, 21, 501–512. [Google Scholar] [CrossRef]
- Liu, Y.; Barks, J.D.; Xu, G.; Silverstein, F.S. Topiramate Extends the Therapeutic Window for Hypothermia-Mediated Neuroprotection after Stroke in Neonatal Rats. Stroke 2004, 35, 1460–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi, L.; Fiorini, P.; Catarzi, S.; Berti, E.; Padrini, L.; Landucci, E.; Donzelli, G.; Bartalena, L.; Fiorentini, E.; Boldrini, A.; et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): A feasibility study. J. Matern. Neonatal Med. 2018, 31, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Alkaitis, M.S.; Crabtree, M.J. Recoupling the Cardiac Nitric Oxide Synthases: Tetrahydrobiopterin Synthesis and Recycling. Curr. Heart Fail. Rep. 2012, 9, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Robertson, C.L.; Scafidi, S.; McKenna, M.C.; Fiskum, G. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp. Neurol. 2009, 218, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomgren, K.; Hagberg, H. Free radicals, mitochondria, and hypoxia–ischemia in the developing brain. Free Radic. Biol. Med. 2006, 40, 388–397. [Google Scholar] [CrossRef]
- Romanowicz, J.; Leonetti, C.; Dhari, Z.; Korotcova, L.; Ramachandra, S.D.; Saric, N.; Morton, P.D.; Bansal, S.; Cheema, A.; Gallo, V.; et al. Treatment with Tetrahydrobiopterin Improves White Matter Maturation in a Mouse Model for Prenatal Hypoxia in Congenital Heart Disease. J. Am. Heart Assoc. 2019, 8, e012711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, J.H.; Tanaka, K.A. Inflammatory response to cardiopulmonary bypass. Ann. Thorac. Surg. 2003, 75, S715–S720. [Google Scholar] [CrossRef]
- Ando, M.; Park, I.-S.; Wada, N.; Takahashi, Y. Steroid Supplementation: A Legitimate Pharmacotherapy after Neonatal Open Heart Surgery. Ann. Thorac. Surg. 2005, 80, 1672–1678. [Google Scholar] [CrossRef]
- Bronicki, R.A.; Backer, C.L.; Baden, H.P.; Mavroudis, C.; Crawford, S.E.; Green, T.P. Dexamethasone reduces the inflammatory response to cardiopulmonary bypass in children. Ann. Thorac. Surg. 2000, 69, 1490–1495. [Google Scholar] [CrossRef]
- Checchia, P.A.; Backer, C.L.; Bronicki, R.A.; Baden, H.P.; Crawford, S.E.; Green, T.P.; Mavroudis, C. Dexamethasone reduces postoperative troponin levels in children undergoing cardiopulmonary bypass*. Crit. Care Med. 2003, 31, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.A.; Pearl, J.M.; Schwartz, S.; Shanley, T.; Manning, P.B.; Nelson, D.P. Combined Steroid Treatment for Congenital Heart Surgery Improves Oxygen Delivery and Reduces Postbypass Inflammatory Mediator Expression. Circulation 2003, 107, 2823–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fudulu, D.P.; Gibbison, B.; Upton, T.; Stoica, S.C.; Caputo, M.; Lightman, S.; Angelini, G.D. Corticosteroids in Pediatric Heart Surgery: Myth or Reality. Front. Pediatr. 2018, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- Graham, E.M.; Martin, R.H.; Buckley, J.R.; Zyblewski, S.C.; Kavarana, M.N.; Bradley, S.M.; Alsoufi, B.; Mahle, W.T.; Hassid, M.; Atz, A.M. Corticosteroid Therapy in Neonates Undergoing Cardiopulmonary Bypass. J. Am. Coll. Cardiol. 2019, 74, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.M.; Atz, A.M.; Butts, R.J.; Baker, N.L.; Zyblewski, S.C.; Deardorff, R.L.; DeSantis, S.M.; Reeves, S.T.; Bradley, S.M.; Spinale, F.G. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: Results from a randomized trial. J. Thorac. Cardiovasc. Surg. 2011, 142, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Zyblewski, S.C.; Martin, R.H.; Shipes, V.B.; Hamlin-Smith, K.; Atz, A.M.; Bradley, S.M.; Kavarana, M.N.; Mahle, W.T.; Everett, A.D.; Graham, E.M. Intraoperative Methylprednisolone and Neurodevelopmental Outcomes in Infants after Cardiac Surgery. Ann. Thorac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.A.; Loukogeorgakis, S.; Yannopoulos, F.; Rimpiläinen, E.; Petzold, A.; Tuominen, H.; Lepola, P.; MacAllister, R.J.; Deanfield, J.; Mäkelä, T.; et al. Remote Ischemic Preconditioning Protects the Brain Against Injury after Hypothermic Circulatory Arrest. Circulation 2011, 123, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, C.; Gao, M.; Dornbos, D.; Ding, Y.; Zeng, X.; Luo, Y.; Ji, X. Remote ischemic post-conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol. Res. 2011, 33, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Gao, X.; Steinberg, G.; Zhao, H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 2008, 151, 1099–1103. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, J.W.; Nicolson, S.C.; Spray, D.M.; Burnham, N.B.; Chittams, J.L.; Sammarco, T.; Walsh, K.W.; Spray, T.L.; Licht, D. Remote Ischemic Preconditioning Does Not Prevent White Matter Injury in Neonates. Ann. Thorac. Surg. 2018, 106, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Gao, Z.; Chen, M.; Zhao, J.; Wang, F.; Li, L.; Dong, H.; Liu, L.; Wang, Q.; Xiong, L. Cardioprotective effect of remote ischemic postconditioning on children undergoing cardiac surgery: A randomized controlled trial. Pediatr. Anesth. 2013, 23, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Velthoven, C.T.; Kavelaars, A.; Heijnen, C.J. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr. Res. 2012, 71, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Sarkislali, K.; Leonetti, C.; Kapani, N.; Dhari, Z.; Al Haj, I.; Ulrey, R.; Hanley, P.J.; Jonas, R.A.; Ishibashi, N. Impact of Mesenchymal Stromal Cell Delivery Through Cardiopulmonary Bypass on Postnatal Neurogenesis. Ann. Thorac. Surg. 2019, 109, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Briggs, C.M.; Datar, A.; Brantner, C.A.; Hanley, P.J.; Jonas, R.A.; Ishibashi, N. Influence of administration of mesenchymal stromal cell on pediatric oxygenator performance and inflammatory response. JTCVS Open 2021, 5, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, V.; Juul, S.E. Erythropoietin: Emerging Role of Erythropoietin in Neonatal Neuroprotection. Pediatr. Neurol. 2014, 51, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, H.; Reibel, N.J.; Bührer, C.; Dame, C. Prophylactic Early Erythropoietin for Neuroprotection in Preterm Infants: A Meta-analysis. Pediatrics 2017, 139, e20164317. [Google Scholar] [CrossRef] [Green Version]
- Juul, S.E.; Comstock, B.A.; Wadhawan, R.; Mayock, D.E.; Courtney, S.E.; Robinson, T.; Ahmad, K.; Bendel-Stenzel, E.; Baserga, M.; LaGamma, E.F.; et al. A Randomized Trial of Erythropoietin for Neuroprotection in Preterm Infants. N. Engl. J. Med. 2020, 382, 233–243. [Google Scholar] [CrossRef]
- Andropoulos, D.B.; Brady, K.; Easley, R.B.; Dickerson, H.A.; Voigt, R.G.; Shekerdemian, L.S.; Meador, M.R.; Eisenman, C.A.; Hunter, J.V.; Turcich, M.; et al. Erythropoietin neuroprotection in neonatal cardiac surgery: A phase I/II safety and efficacy trial. J. Thorac. Cardiovasc. Surg. 2013, 146, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Diaz, L.K.; Gaynor, J.W.; Koh, S.J.; Ittenbach, R.F.; Gerdes, M.; Bernbaum, J.C.; Zackai, E.H.; Clancy, R.R.; Rehman, M.A.; Pennington, J.W.; et al. Increasing cumulative exposure to volatile anesthetic agents is associated with poorer neurodevelopmental outcomes in children with hypoplastic left heart syndrome. J. Thorac. Cardiovasc. Surg. 2016, 152, 482–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, G.G.; Robertson, C.M.T.; Alton, G.Y.; Joffe, A.R.; Cave, D.A.; Dinu, I.A.; Creighton, D.E.; Ross, D.B.; Rebeyka, I.M.; The Western Canadian Complex Pediatric Therapies Follow-Up Group. Neurodevelopmental outcome following exposure to sedative and analgesic drugs for complex cardiac surgery in infancy*. Pediatr. Anesth. 2011, 21, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Zuppa, A.F.; Nicolson, S.C.; Wilder, N.S.; Ibla, J.C.; Gottlieb, E.A.; Burns, K.M.; Stylianou, M.; Trachtenberg, F.; Ni, H.; Skeen, T.H.; et al. Results of a phase 1 multicentre investigation of dexmedetomidine bolus and infusion in corrective infant cardiac surgery. Br. J. Anaesth. 2019, 123, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Ming, S.; Xie, Y.; Du, X.; Huang, H.; Fan, Y.; Liang, Q.; Xie, Y. Effect of dexmedetomidine on perioperative hemodynamics and organ protection in children with congenital heart disease. Medicine 2021, 100, e23998. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, R.; Shen, L.; Xie, Y.; Li, X. The brain protective effect of dexmedetomidine during surgery for paediatric patients with congenital heart disease. J. Int. Med. Res. 2019, 47, 1677–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, F.; Gastonguay, M.R.; Nicolson, S.C.; DiLiberto, M.; Ocampo-Pelland, A.; Zuppa, A.F. Dexmedetomidine Pharmacology in Neonates and Infants after Open Heart Surgery. Anesth. Analg. 2016, 122, 1556–1566. [Google Scholar] [CrossRef]
- Sonneville, R.; Hertog, H.M.D.; Güiza, F.; Gunst, J.; Derese, I.; Wouters, P.J.; Brouland, J.-P.; Polito, A.; Gray, F.; Chrétien, F.; et al. Impact of Hyperglycemia on Neuropathological Alterations during Critical Illness. J. Clin. Endocrinol. Metab. 2012, 97, 2113–2123. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A.; Morley, R.; Cole, T.J. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ 1988, 297, 1304–1308. [Google Scholar] [CrossRef] [Green Version]
- Sadhwani, A.; Asaro, L.; Goldberg, C.; Ware, J.; Butcher, J.; Gaies, M.; Smith, C.; Alexander, J.L.; Wypij, D.; Agus, M.S. Impact of Tight Glycemic Control on Neurodevelopmental Outcomes at 1 Year of Age for Children with Congenital Heart Disease: A Randomized Controlled Trial. J. Pediatr. 2016, 174, 193–198.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, M.S.; Asaro, L.; Steil, G.M.; Alexander, J.L.; Silverman, M.; Wypij, D.; Gaies, M.G. Tight Glycemic Control after Pediatric Cardiac Surgery in High-Risk Patient Populations. Circulation 2014, 129, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Epstein, F.H.; Mccord, J.M. Oxygen-Derived Free Radicals in Postischemic Tissue Injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Towfighi, J.; Roberts, R.L.; Heitjan, D.F. Allopurinol Administered after Inducing Hypoxia-Ischemia Reduces Brain Injury in 7-Day-Old Rats. Pediatr. Res. 1993, 33, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Gunes, T.; Ozturk, M.A.; Koklu, E.; Kose, K.; Gunes, I. Effect of Allopurinol Supplementation on Nitric Oxide Levels in Asphyxiated Newborns. Pediatr. Neurol. 2007, 36, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, J.J.; Van Bel, F.; Veen, S.; Derks, J.B.; Groenendaal, F.; Rijken, M.; Roze, E.; Venema, M.M.A.U.; Rademaker, C.M.A.; Bos, A.F.; et al. Long-term neuroprotective effects of allopurinol after moderate perinatal asphyxia: Follow-up of two randomised controlled trials. Arch. Dis. Child. Fetal Neonatal. Ed. 2012, 97, F162–F166. [Google Scholar] [CrossRef]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Sheu, S.-S.; Nauduri, D.; Anders, M. Targeting antioxidants to mitochondria: A new therapeutic direction. Biochim. Biophys. Acta Mol. Basis Dis. 2006, 1762, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.H.; Matei, N.; Camara, R. Emerging mechanisms and novel applications of hydrogen gas therapy. Med. Gas Res. 2018, 8, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jia, L.; Chen, B.; Zhang, L.; Liu, J.; Long, J.; Li, Y. Hydrogen Inhalation is Superior to Mild Hypothermia in Improving Cardiac Function and Neurological Outcome in an Asphyxial Cardiac Arrest Model of Rats. Shock 2016, 46, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.R.; Perry, D.A.; Raza, A.; Nedder, A.P.; Pollack, E.; Regan, W.L.; Bosch, S.J.V.D.; Polizzotti, B.D.; Yang, E.; Davila, D.; et al. Perioperatively Inhaled Hydrogen Gas Diminishes Neurologic Injury Following Experimental Circulatory Arrest in Swine. JACC Basic Transl. Sci. 2019, 4, 176–187. [Google Scholar] [CrossRef]
- Kajimoto, M.; Nuri, M.; Sleasman, J.R.; Charette, K.A.; Nelson, B.R.; Portman, M.A. Inhaled nitric oxide reduces injury and microglia activation in porcine hippocampus after deep hypothermic circulatory arrest. J. Thorac. Cardiovasc. Surg. 2021, 161, e485–e498. [Google Scholar] [CrossRef]
- Checchia, P.A.; Bronicki, R.A.; Muenzer, J.T.; Dixon, D.; Raithel, S.; Gandhi, S.; Huddleston, C.B. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children—A randomized trial. J. Thorac. Cardiovasc. Surg. 2013, 146, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, C.; Millar, J.; Horton, S.; Brizard, C.; Molesworth, C.; Butt, W. Nitric oxide administration during paediatric cardiopulmonary bypass: A randomised controlled trial. Intensiv. Care Med. 2016, 42, 1744–1752. [Google Scholar] [CrossRef]
- Curvello, V.; Pastor, P.; Hekierski, H.; Armstead, W.M. Inhaled Nitric Oxide Protects Cerebral Autoregulation and Reduces Hippocampal Necrosis after Traumatic Brain Injury Through Inhibition of ET-1, ERK MAPK and IL-6 Upregulation in Pigs. Neurocrit Care 2018, 30, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Uryash, A.; Bassuk, J.; Kurlansky, P.; Giridharan, G.A.; Shakeri, M.; Estrada, R.; Sethu, P.; Adams, J.A. Mechanisms of Periodic Acceleration Induced Endothelial Nitric Oxide Synthase (eNOS) Expression and Upregulation Using an In Vitro Human Aortic Endothelial Cell Model. Cardiovasc. Eng. Technol. 2012, 3, 292–301. [Google Scholar] [CrossRef]
- Endres, M.; Laufs, U.; Liao, J.K.; Moskowitz, M.A. Targeting eNOS for stroke protection. Trends Neurosci. 2004, 27, 283–289. [Google Scholar] [CrossRef]
- Adams, J.A.; Pastuszko, P.; Uryash, A.; Wilson, D.; Padrino, J.R.L.; Nadkarni, V.; Pastuszko, A. Whole Body Periodic Acceleration (pGz) as a non-invasive preconditioning strategy for pediatric cardiac surgery. Med. Hypotheses 2018, 110, 144–149. [Google Scholar] [CrossRef]
- Yu, H.; Shi, L.; Zhao, S.; Sun, Y.; Gao, Y.; Sun, Y.; Qi, G. Triptolide Attenuates Myocardial Ischemia/Reperfusion Injuries in Rats by Inducing the Activation of Nrf2/HO-1 Defense Pathway. Cardiovasc. Toxicol. 2015, 16, 325–335. [Google Scholar] [CrossRef]
- Hao, M.; Li, X.; Feng, J.; Pan, N. Triptolide Protects Against Ischemic Stroke in Rats. Inflammation 2015, 38, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; Jin, X.-Q.; Ye, F.; Zhao, Y.; Zhang, J.-J. Triptolide attenuates cerebral ischemia and reperfusion injury in rats through the inhibition the nuclear factor kappa B signaling pathway. Neuropsychiatr. Dis. Treat. 2015, 11, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-F.; Lee, T.-S.; Kou, Y.R. Anti-inflammatory and neuroprotective effects of triptolide on traumatic brain injury in rats. Respir. Physiol. Neurobiol. 2012, 182, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lei, Y.-Q.; Liu, J.-F.; Wang, Z.-C.; Cao, H. Triptolide improves neurobehavioral functions, inflammation, and oxidative stress in rats under deep hypothermic circulatory arrest. Aging 2021, 13, 3031–3044. [Google Scholar] [CrossRef]
- Romero-Miguel, D.; Lamanna-Rama, N.; Casquero-Veiga, M.; Gómez-Rangel, V.; Desco, M.; Soto-Montenegro, M.L. Minocycline in neurodegenerative and psychiatric diseases: An update. Eur. J. Neurol. 2021, 28, 1056–1081. [Google Scholar] [CrossRef]
- Drabek, T.; Janata, A.; Wilson, C.D.; Stezoski, J.; Janesko-Feldman, K.; Tisherman, S.A.; Foley, L.M.; Verrier, J.D.; Kochanek, P.M. Minocycline attenuates brain tissue levels of TNF-α produced by neurons after prolonged hypothermic cardiac arrest in rats. Resuscitation 2014, 85, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Salameh, A.; Einenkel, A.; Kühne, L.; Grassl, M.; Von Salisch, S.; Kiefer, P.; Vollroth, M.; Dähnert, I.; Dhein, S. Hippocampal Neuroprotection by Minocycline and Epigallo-Catechin-3-Gallate against Cardiopulmonary Bypass-Associated Injury. Brain Pathol. 2015, 25, 733–742. [Google Scholar] [CrossRef]
- Buller, K.M.; Carty, M.L.; Reinebrant, H.; Wixey, J. Minocycline: A neuroprotective agent for hypoxic-ischemic brain injury in the neonate? J. Neurosci. Res. 2009, 87, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ares, S.; Saenz-Rico, B.; Quero, J.; De Escobar, G.M. Iodine and the Effects on Growth in Premature Newborns: A Focus on the Role of Thyroid Hormones in Neurodevelopment and Growth. In Handbook of Growth and Growth Monitoring in Health and Disease; Springer: New York, NY, USA, 2012. [Google Scholar]
- Calaciura, F.; Mendorla, G.; Distefano, M.; Castorina, S.; Fario, T.; Motta, R.M.; Sava, L.; Delange, F.; Vigneri, R. Childhood IQ measurements in infants with transient congenital hypothyroidism. Clin. Endocrinol. 1995, 43, 473–477. [Google Scholar] [CrossRef]
- Plumpton, K.; Haas, N.A. Identifying infants at risk of marked thyroid suppression post-cardiopulmonary bypass. Intensiv. Care Med. 2005, 31, 581–587. [Google Scholar] [CrossRef]
- Dimmick, S.J.; Badawi, N.; Randell, T. Thyroid hormone supplementation for the prevention of morbidity and mortality in infants undergoing cardiac surgery. Cochrane Database Syst. Rev. 2004, CD004220. [Google Scholar] [CrossRef] [PubMed]
- Bettendorf, M.; Schmidt, K.G.; Grulich-Henn, J.; Ulmer, H.E.; Heinrich, U.E. Tri-iodothyronine treatment in children after cardiac surgery: A double-blind, randomised, placebo-controlled study. Lancet 2000, 356, 529–534. [Google Scholar] [CrossRef]
- Mittnacht, J.; Choukair, D.; Kneppo, C.; Brunner, R.; Parzer, P.; Gorenflo, M.; Bettendorf, M. Long-Term Neurodevelopmental Outcome of Children Treated with Tri-Iodothyronine after Cardiac Surgery: Follow-Up of a Double-Blind, Randomized, Placebo-Controlled Study. Horm. Res. Paediatr. 2015, 84, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soshi, T.; Andersson, M.; Kawagoe, T.; Nishiguchi, S.; Yamada, M.; Otsuka, Y.; Nakai, R.; Abe, N.; Aslah, A.; Igasaki, T.; et al. Prefrontal Plasticity after a 3-Month Exercise Intervention in Older Adults Relates to Enhanced Cognitive Performance. Cereb. Cortex 2021, 31, 4501–4517. [Google Scholar] [CrossRef] [PubMed]
- Wilckens, K.A.; Stillman, C.M.; Waiwood, A.M.; Kang, C.; Leckie, R.L.; Peven, J.C.; Foust, J.E.; Fraundorf, S.H.; Erickson, K.I. Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus 2021, 31, 335–347. [Google Scholar] [CrossRef]
- Rovio, S.; Spulber, G.; Nieminen, L.J.; Niskanen, E.; Winblad, B.; Tuomilehto, J.; Nissinen, A.; Soininen, H.; Kivipelto, M. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol. Aging 2010, 31, 1927–1936. [Google Scholar] [CrossRef]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [Green Version]
- de Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef]
- Dulfer, K.; Duppen, N.; Kuipers, I.M.; Schokking, M.; van Domburg, R.T.; Verhulst, F.C.; Helbing, W.A.; Utens, E.M. Aerobic Exercise Influences Quality of Life of Children and Youngsters with Congenital Heart Disease: A Randomized Controlled Trial. J. Adolesc. Health 2014, 55, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Fourdain, S.; Simard, M.-N.; Dagenais, L.; Materassi, M.; Doussau, A.; Goulet, J.; Gagnon, K.; Prud’Homme, J.; Vinay, M.-C.; Dehaes, M.; et al. Gross Motor Development of Children with Congenital Heart Disease Receiving Early Systematic Surveillance and Individualized Intervention: Brief Report. Dev. Neurorehabilit. 2021, 24, 56–62. [Google Scholar] [CrossRef]
- Dam, J.C.; van, E.-V.; Vlieland, T.P.M.V.; Kuipers, I.M.; Blom, N.A.; Harkel, A.D.J.T. Improvement of physical activity levels in children and adolescents after surgery for congenital heart disease: Preferences and use of physical therapy. Disabil. Rehabil. 2021, 1–8. [Google Scholar] [CrossRef]
- Calderon, J.; Wypij, D.; Rofeberg, V.; Stopp, C.; Roseman, A.; Albers, D.; Newburger, J.W.; Bellinger, D.C. Randomized Controlled Trial of Working Memory Intervention in Congenital Heart Disease. J. Pediatr. 2020, 227, 191–198.e3. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.; Fernell, E.; Olesen, J.; Johnson, M.; Gustafsson, P.; Dahlström, K.; Gillberg, C.G.; Forssberg, H.; Westerberg, H. Computerized Training of Working Memory in Children with ADHD-A Randomized, Controlled Trial. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewaldt, K.H.; Løhaugen, G.C.C.; Austeng, D.; Brubakk, A.-M.; Skranes, J. Working Memory Training Improves Cognitive Function in VLBW Preschoolers. Pediatrics 2013, 131, e747–e754. [Google Scholar] [CrossRef] [Green Version]
- Løhaugen, G.C.; Antonsen, I.; Håberg, A.; Gramstad, A.; Vik, T.; Brubakk, A.-M.; Skranes, J. Computerized Working Memory Training Improves Function in Adolescents Born at Extremely Low Birth Weight. J. Pediatr. 2011, 158, 555–561.e4. [Google Scholar] [CrossRef]
- Nampijja, M.; Kizindo, R.; Apule, B.; Lule, S.; Muhangi, L.; Titman, A.; Elliott, A.; Alcock, K.; Lewis, C. The role of the home environment in neurocognitive development of children living in extreme poverty and with frequent illnesses: A cross-sectional study. Wellcome Open Res. 2018, 3, 152. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.; Orton, J.; Anderson, P.; Boyd, R.; Doyle, L. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. 2015, 11, CD005495. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.M.; Patel, A.L.; Meier, P.; Patra, K. Maternal Education Level Predicts Cognitive, Language, and Motor Outcome in Preterm Infants in the Second Year of Life. Am. J. Perinatol. 2016, 33, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Mackes, N.K.; Golm, D.; Sarkar, S.; Kumsta, R.; Rutter, M.; Fairchild, G.; Mehta, M.A.; Sonuga-Barke, E.J.S.; Era Young Adult Follow-Up on behalf of the ERA Young Adult Follow-up team. Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc. Natl. Acad. Sci. USA 2020, 117, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Bonthrone, A.F.; Chew, A.; Kelly, C.J.; Almedom, L.; Simpson, J.; Victor, S.; Edwards, A.D.; Rutherford, M.A.; Nosarti, C.; Counsell, S.J. Cognitive function in toddlers with congenital heart disease: The impact of a stimulating home environment. Infancy 2021, 26, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Limperopoulos, C.; Majnemer, A.; Shevell, M.I.; Rohlicek, C.; Rosenblatt, B.; Tchervenkov, C.; Darwish, H. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J. Pediatr. 2002, 141, 51–58. [Google Scholar] [CrossRef]
- Forbess, J.M.; Visconti, K.J.; Hancock-Friesen, C.; Howe, R.C.; Bellinger, D.C.; Jonas, R.A. Neurodevelopmental Outcome after Congenital Heart Surgery: Results from an Institutional Registry. Circulation 2002, 106, I-95. [Google Scholar] [CrossRef]
- Atallah, J.; Dinu, I.A.; Joffe, A.R.; Robertson, C.M.T.; Sauve, R.S.; Dyck, J.D.; Ross, D.B.; Rebeyka, I.M.; Western the Western Canadian Complex Pediatric Therapies Follow-Up Group. Two-Year Survival and Mental and Psychomotor Outcomes after the Norwood Procedure. Circulation 2008, 118, 1410–1418. [Google Scholar] [CrossRef]
- Gaynor, J.W.; Stopp, C.; Wypij, D.; Andropoulos, D.B.; Atallah, J.; Atz, A.M.; Beca, J.; Donofrio, M.T.; Duncan, K.; Ghanayem, N.S.; et al. Neurodevelopmental Outcomes after Cardiac Surgery in Infancy. Pediatrics 2015, 135, 816–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Stage of Patient Care | Type of Study | Treatment | References | Clinical Trial |
|---|---|---|---|---|
| Maternal | Clinical | Maternal Hyperoxia | [45,46,47] | https://clinicaltrials.gov/ct2/show/NCT03944837, accessed on 3 November 2021 |
| Progesterone | [48,49,50,51,52] | https://clinicaltrials.gov/ct2/show/NCT02133573, accessed on 3 November 2021 | ||
| Topiramate Prophylaxis | [53,54,55,56,57,58] | https://clinicaltrials.gov/ct2/show/NCT01426542, accessed on 3 November 2021 | ||
| Pre-clinical | Tetrahydrobiopterine | [59,60,61,62] | n/a | |
| Perioperative | Clinical | Corticosteroids | [63,64,65,66,67,68,69,70,71] | https://clinicaltrials.gov/ct2/show/NCT03229538, accessed on 3 November 2021 |
| Remote Ischemic Preconditioning | [72,73,74,75,76] | https://clinicaltrials.gov/ct2/show/NCT01835392, accessed on 3 November 2021 | ||
| Mesenchymal Stromal Cells | [77,78,79,80] | https://clinicaltrials.gov/ct2/show/NCT04236479, accessed on 3 November 2021 | ||
| Erythropoietin | [81,82,83,84] | n/a | ||
| Dexmedetomidine | [85,86,87,88,89,90] | https://clinicaltrials.gov/ct2/show/NCT02492269, accessed on 3 November 2021 | ||
| https://clinicaltrials.gov/ct2/show/NCT03882788, accessed on 3 November 2021 | ||||
| https://clinicaltrials.gov/ct2/show/NCT02492269, accessed on 3 November 2021 | ||||
| https://clinicaltrials.gov/ct2/show/NCT03366597, accessed on 3 November 2021 | ||||
| https://clinicaltrials.gov/ct2/show/NCT04484922, accessed on 3 November 2021 | ||||
| Tight Glycemic Control | [91,92,93,94] | n/a | ||
| Alloprinol | [95,96,97,98] | https://clinicaltrials.gov/ct2/show/NCT04217421, accessed on 3 November 2021 | ||
| Pre-clinical | Inhaled H2 | [99,100,101,102,103] | n/a | |
| Inhaled Nitric Oxide | [104,105,106,107] | n/a | ||
| Whole Body Periodic Acceleration | [108,109,110] | n/a | ||
| Triptoide | [111,112,113,114,115] | n/a | ||
| Minocycline | [116,117,118,119] | n/a | ||
| Postoperative | Clinical | Triiodothyronine | [120,121,122,123,124,125] | n/a |
| Standardized Exercise Program | [126,127,128,129,130,131,132,133] | https://clinicaltrials.gov/ct2/show/NCT02542683, accessed on 3 November 2021 | ||
| Physical Therapy | [134,135] | n/a | ||
| Cogmed Working Memory Training | [136,137,138,139] | https://clinicaltrials.gov/ct2/show/NCT03023644, accessed on 3 November 2021 | ||
| Early Stimulation | [140,141,142,143] | https://clinicaltrials.gov/ct2/show/NCT04152330, accessed on 3 November 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, K.; Liu, C.; Jonas, R.A.; Ishibashi, N. The Current Status of Neuroprotection in Congenital Heart Disease. Children 2021, 8, 1116. https://0-doi-org.brum.beds.ac.uk/10.3390/children8121116
Kobayashi K, Liu C, Jonas RA, Ishibashi N. The Current Status of Neuroprotection in Congenital Heart Disease. Children. 2021; 8(12):1116. https://0-doi-org.brum.beds.ac.uk/10.3390/children8121116
Chicago/Turabian StyleKobayashi, Kei, Christopher Liu, Richard A. Jonas, and Nobuyuki Ishibashi. 2021. "The Current Status of Neuroprotection in Congenital Heart Disease" Children 8, no. 12: 1116. https://0-doi-org.brum.beds.ac.uk/10.3390/children8121116