Long-Term Outcome of Indomethacin Treatment in Pediatric Patients with Paroxysmal Hemicrania—A Case Series
Abstract
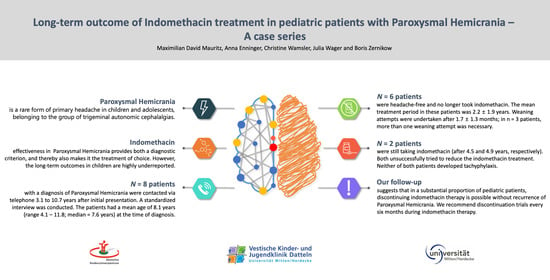
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Blankenburg, M.; Hechler, T.; Dubbel, G.; Wamsler, C.; Zernikow, B. Paroxysmal hemicrania in children—Symptoms, diagnostic criteria, therapy and outcome. Cephalalgia 2008, 29, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Lambru, G.; Byrne, S. Trigeminal autonomic cephalalgias in children and adolescents. Neurol. Sci. 2018, 39. [Google Scholar] [CrossRef] [PubMed]
- Frusciante, R.; Capuano, A.; Vollono, C.; Vigevano, F.; Tarantino, S.; Valeriani, M. P047. Paroxysmal episodic hemicrania in a child. A complex differential diagnosis. J. Headache Pain 2015, 16, A100. [Google Scholar] [CrossRef] [Green Version]
- Evers, S.; Summ, O.; Frese, A. Very young age of onset in trigemino-autonomic cephalalgias—Case report and review of the literature. Cephalalgia 2020, 40, 1385–1388. [Google Scholar] [CrossRef]
- Baraldi, C.; Pellesi, L.; Guerzoni, S.; Cainazzo, M.M.; Pini, L.A. Therapeutical approaches to paroxysmal hemicrania, hemicrania continua and short lasting unilateral neuralgiform headache attacks: A critical appraisal. J. Headache Pain 2017, 18, 71. [Google Scholar] [CrossRef]
- Ramusino, M.; Perini, G.; Antonaci, F.; Costa, A. The treatment of trigeminal autonomic cephalalgias: An overview. J. Oral Facial Pain H 2019, 33, 89–104. [Google Scholar] [CrossRef]
- Wei, D.Y.; Jensen, R.H. Therapeutic approaches for the management of trigeminal autonomic cephalalgias. Neurotherapeutics 2018, 15, 346–360. [Google Scholar] [CrossRef] [Green Version]
- Giani, L.; Cecchini, A.P.; Leone, M. Cluster headache and TACs: State of the art. Neurol. Sci. 2020, 41. [Google Scholar] [CrossRef]
- Barloese, M.C.J. The pathophysiology of the trigeminal autonomic cephalalgias, with clinical implications. Clin. Auton. Res. 2018, 28, 315–324. [Google Scholar] [CrossRef]
- Summ, O.; Andreou, A.P.; Akerman, S.; Holland, P.R.; Hoffmann, J.; Goadsby, P.J. Differential actions of indomethacin: Clinical relevance in headache. Pain 2020. [Google Scholar] [CrossRef] [PubMed]
- Summ, O.; Andreou, A.P.; Akerman, S.; Goadsby, P.J. A potential nitrergic mechanism of action for indomethacin, but not of other COX inhibitors: Relevance to indomethacin-sensitive headaches. J. Headache Pain 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Matharu, M.S.; Goadsby, P.J. Trigeminal autonomic cephalalgias: Current and future treatments. Headache J. Head Face Pain 2007, 47, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Boes, C.J.; Dodick, D.W. Refining the clinical spectrum of chronic paroxysmal hemicrania: A review of 74 patients. Headache J. Head Face Pain 2002, 42, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Sjaastad, O.; Antonaci, F. Chronic paroxysmal hemicrania: A case report. Cephalalgia 1987, 7, 203–205. [Google Scholar] [CrossRef]
- Jensen, N.B.; Joensen, P.; Jensen, J. Chronic paroxysmal hemicrania: Continued remission of symptoms after discontinuation of indomethacin. Cephalalgia 1982, 2, 163–164. [Google Scholar] [CrossRef]
- Ishii, R.; Fukazawa, R.; Takezawa, H.; Fujii, A. Case report: Shortest course of pediatric paroxysmal hemicrania. Headache J. Head Face Pain 2019, 59, 1084–1087. [Google Scholar] [CrossRef] [Green Version]
- Pareja, J.; Caminero, A.; Franco, E.; Casado, J.; Pascual, J.; del Dose Río, M.S. Efficacy and tolerability of long-term indomethacin treatment of chronic paroxysmal hemicrania and hemicrania continua. Cephalalgia 2001, 21, 906–910. [Google Scholar] [CrossRef]
- Hoppmann, R.A.; Peden, J.G.; Ober, S.K. Central nervous system side effects of nonsteroidal anti-inflammatory drugs: Aseptic meningitis, psychosis, and cognitive dysfunction. Arch. Intern. Med. 1991, 151, 1309–1313. [Google Scholar] [CrossRef]
- Clunie, M.; Crone, L.-A.; Klassen, L.; Yip, R. Psychiatric side effects of indomethacin in parturients. Can J. Anesth. 2003, 50, 586–588. [Google Scholar] [CrossRef]
- Sehgal, A. Global shortage and rationing of indomethacin: Need to refine approach. J. Perinatol. 2010, 30, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Raieli, V.; Cicala, V.; Vanadia, F. Pediatric paroxysmal hemicrania: A case report and some clinical considerations. Neurol. Sci. 2015, 36, 2295–2296. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, W.P.; Agrawal, S. Management of children and young people with headache. Arch. Dis. Child Educ Pr. Ed. 2017, 102, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Diagnostic Criteria |
|---|
| A. At least 20 attacks fulfilling criteria B–E |
| B. Severe unilateral orbital, supraorbital, and/or temporal pain lasting 2–30 min |
| C. Either or both of the following: |
| 1. At least one of the following symptoms or signs, ipsilateral to the headache: |
| (a) conjunctival injection and/or lacrimation |
| (b) nasal congestion and/or rhinorrhea |
| (c) eyelid edema |
| (d) forehead and facial sweating |
| (e) miosis and/or ptosis |
| 2. A sense of restlessness or agitation |
| D. Occurring with a frequency of > 5 per day 1 |
| E. Prevented absolutely by therapeutic doses of indomethacin |
| F. Not better accounted for by another ICHD-3 diagnosis |
| Item | Patient No./Gender | All Mean ± SD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1/m | 2/m | 3/f | 4/m | 5/m | 6/m | 7/f | 8/m | ||
| Age at PH onset (years) | 1.2 | 5.8 | 6.8 | 7.0 | 7.3 | 7.9 | 10.4 | 11.8 | 7.3 ± 3.2 |
| Age at the time of diagnosis (years) | 4.1 | 6.0 | 7.3 | 7.3 | 7.9 | 9.3 | 10.7 | 11..9 | 8.1 ± 2.5 |
| Time to diagnosis (months) | 34.5 | 2.5 | 6.3 | 3.3 | 8.0 | 17.7 | 3.7 | 7.7 | 10.5 ± 10.8 |
| Mean pain intensity (NRS) | 10 | 6 | 10 | 7 | 6 | 5 | 4 | 10 | 7.3 ± 2.5 |
| Maximum pain intensity (NRS) | 10 | 8 | 10 | 9 | 8 | 10 | 7 | 10 | 9.0 ± 1.2 |
| Mean attack duration (min) | 25 | 20 | 30 | 30 | 30 | 15 | 5 | 3 | 19.8 ± 11.1 |
| Type of PH | EPH | CPH | CPH | CPH | EPH | CPH | CPH | EPH | CPH:EPH ratio 1.67:1 |
| Indomethacin starting dose (mg/kgbw) | 3.0 | 2.96 | 1.33 | 3.41 | 2.36 | 2.82 | 2.65 | 3.35 | 2.75 ± 0.66 |
| Indomethacin treatment period (years) | - | 2.4 | - | 4.2 | 0.9 | 4.6 | 0.4 | 0.5 | 2.2 ± 1.9 |
| ICHD-3 Diagnostic Criteria for PH | Patient No./Gender | All (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1/m | 2/m | 3/f | 4/m | 5/m | 6/m | 7/f | 8/m | |||
| A | At least 20 attacks fulfilling the criteria | x | x | x | x | x | x | x | x | 100 |
| B | Severe unilateral orbital, supraorbital, and/or temporal pain lasting 2–30 min | x | x | x | x | x | x | x | x | 100 |
| C | Either or both of the following: | |||||||||
| 1. Headache accompanied by (at least one) | ||||||||||
| (a) conjunctival injection and/or lacrimation | x | x | 25.0 | |||||||
| (b) nasal congestion and/or rhinorrhea | x | x | x | 37.5 | ||||||
| (c) eyelid edema | 0 | |||||||||
| (d) forehead and facial sweating | x | x | x | x | 50.0 | |||||
| (e) miosis and/or ptosis | x | x | x | 37.5 | ||||||
| 2. A sense of restlessness or agitation | x | x | x | x | x | 62.5 | ||||
| D | Occurring with a frequency of > 5 per day 1 | x | x | x | x | x | x | x | x | 100 |
| E | Prevented absolutely by indomethacin | x | x | x | x | x | x | x | x | 100 |
| F | Not better accounted for by other diagnosis | x | x | x | x | x | x | x | x | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauritz, M.D.; Enninger, A.; Wamsler, C.; Wager, J.; Zernikow, B. Long-Term Outcome of Indomethacin Treatment in Pediatric Patients with Paroxysmal Hemicrania—A Case Series. Children 2021, 8, 101. https://0-doi-org.brum.beds.ac.uk/10.3390/children8020101
Mauritz MD, Enninger A, Wamsler C, Wager J, Zernikow B. Long-Term Outcome of Indomethacin Treatment in Pediatric Patients with Paroxysmal Hemicrania—A Case Series. Children. 2021; 8(2):101. https://0-doi-org.brum.beds.ac.uk/10.3390/children8020101
Chicago/Turabian StyleMauritz, Maximilian David, Anna Enninger, Christine Wamsler, Julia Wager, and Boris Zernikow. 2021. "Long-Term Outcome of Indomethacin Treatment in Pediatric Patients with Paroxysmal Hemicrania—A Case Series" Children 8, no. 2: 101. https://0-doi-org.brum.beds.ac.uk/10.3390/children8020101