A Multivariate Pattern Analysis of Metabolic Profile in Neurologically Impaired Children and Adolescents
Abstract
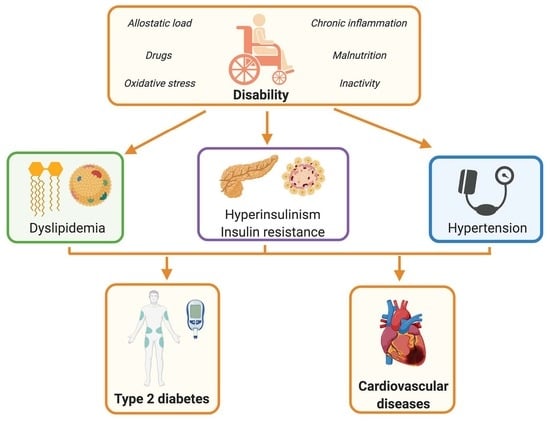
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
2.2.1. Anthropometric Parameters and Body Composition
2.2.2. Biochemical and Endocrinological Parameters
3. Statistical Analysis
4. Results
4.1. Univariate Analysis
4.2. Multivariate Analysis
4.3. Logistic Regression Model
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Sherling, D.H.; Perumareddi, P.; Hennekens, C.H. Metabolic Syndrome: Clinical and Policy Implications of the New Silent Killer. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 365–367. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, V. Metabolic syndrome: What are the risks for humans? Biosci. Trends 2010, 4, 204–212. [Google Scholar]
- Gluvic, Z.; Zaric, B.; Resanovic, I.; Obradovic, M.; Mitrovic, A.; Radak, D.; Isenovic, E. Link between Metabolic Syndrome and Insulin Resistance. Curr. Vasc. Pharmacol. 2016, 15, 30–39. [Google Scholar] [CrossRef]
- Wittcopp, C.; Conroy, R. Metabolic Syndrome in Children and Adolescents. Pediatr. Rev. 2016, 37, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal pro-gramming of the metabolic syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Bussler, S.; Penke, M.; Flemming, G.; Elhassan, Y.S.; Kratzsch, J.; Sergeyev, E.; Lipek, T.; Vogel, M.; Spielau, U.; Körner, A.; et al. Novel Insights in the Metabolic Syndrome in Childhood and Adolescence. Horm. Res. Paediatr. 2017, 88, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, C.; Nkeh-Chungag, B.N.; Fredriksen, P.M.; Goswami, N. The prevalence of pediatric metabolic syndrome—A critical look on the discrepancies between definitions and its clinical importance. Int. J. Obes. 2021, 45, 12–24. [Google Scholar] [CrossRef]
- DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. [Google Scholar] [CrossRef] [Green Version]
- Pérez, E.A.; Olivares, V.M.; Martínez-Espinosa, R.M.; Vila, M.D.M.; García-Galbis, M.R. New Insights about How to Make an Intervention in Children and Adolescents with Metabolic Syndrome: Diet, Exercise vs. Changes in Body Composition. A Systematic Review of RCT. Nutrients 2018, 10, 878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcaterra, V.; Klersy, C.; Muratori, T.; Telli, S.; Caramagna, C.; Scaglia, F.; Cisternino, M.; Larizza, D. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clin. Endocrinol. 2008, 68, 868–872. [Google Scholar] [CrossRef]
- Friend, A.; Craig, L.; Turner, S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Agudelo, G.M.; Bedoya, G.; Estrada, A.F.; Patiño, F.A.; Muñoz, A.M.; Velásquez, C.M. Variations in the Prevalence of Metabolic Syndrome in Adolescents According to Different Criteria Used for Diagnosis: Which Definition Should Be Chosen for This Age Group? Metab. Syndr. Relat. Disord. 2014, 12, 202–209. [Google Scholar] [CrossRef]
- Pelizzo, G.; Calcaterra, V.; Carlini, V.; Fusillo, M.; Manuelli, M.; Klersy, C.; Pasqua, N.; Luka, E.; Albertini, R.; De Amici, M.; et al. Nutritional status and metabolic profile in neurologically impaired pediatric surgical patients. J. Pediatr. Endocrinol. Metab. 2017, 30, 289–300. [Google Scholar] [CrossRef]
- Pelizzo, G.; Calcaterra, V.; Acierno, C.; Cena, H. Malnutrition and associated risk factors among disabled children. Special considerations in the pediatric surgical “fragile” patients. Front. Pediatr. 2019, 7, 86. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Gao, T.; Zhong, F.; Cai, J.; Sun, Y.; Ma, A. Association between Sarcopenia and Metabolic Syndrome in Middle-Aged and Older Non-Obese Adults: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, P.A.B.; Sacramento, A.D.J.; Carmo, C.I.D.D.D.; Silva, L.B.; Silqueira, S.M.D.F.; Soares, S.M. Factors associated with metabolic syndrome in older adults: A population-based study. Rev. Bras. Enferm. 2019, 72, 221–228. [Google Scholar] [CrossRef] [Green Version]
- June-Young, L.; Lee, J.-Y.; Kim, D.-H. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly. Medicine 2017, 96, e8491. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; De Silvestri, A.; Albertini, R.; De Amici, M.; Valenza, M.; Pelizzo, G. Stress Measured by Allostatic Load in Neurologically Impaired Children: The Importance of Nutritional Status. Horm. Res. Paediatr. 2017, 88, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Cena, H.; De Silvestri, A.; Girgenti, V.; Bommarito, D.; Pelizzo, G. Diabetes Type 2 in Neurologically Impaired Children and Adolescents Without Obesity: A New Emerging Entity? Front. Neurol. 2019, 10, 947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, D.; Shavelle, R.; Reynolds, R.; Rosenbloom, L.; Day, S. Survival in cerebral palsy in the last 20 years: Signs of improvement? Dev. Med. Child Neurol. 2007, 49, 86–92. [Google Scholar] [CrossRef]
- Smithers-Sheedy, H.; Badawi, N.; Blair, E.; Cans, C.; Himmelmann, K.; Krägeloh-Mann, I.; Mcintyre, S.; Slee, J.; Uldall, P.; Watson, L.; et al. What constitutes cerebral palsy in the twenty-first century? Dev. Med. Child Neurol. 2014, 56, 823–828. [Google Scholar] [CrossRef]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- Rajalahti, T.; Kroksveen, A.C.; Arneberg, R.; Berven, F.S.; Vedeler, C.A.; Myhr, K.-M.; Kvalheim, O.M. A Multivariate Approach to Reveal Biomarker Signatures for Disease Classification: Application to Mass Spectral Profiles of Cerebrospinal Fluid from Patients with Multiple Sclerosis. J. Proteome Res. 2010, 9, 3608–3620. [Google Scholar] [CrossRef] [PubMed]
- Rajalahti, T.; Kvalheim, O.M. Multivariate data analysis in pharmaceutics: A tutorial review. Int. J. Pharm. 2011, 417, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child Neurol. 2008, 39, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Calcaterra, V.; Montalbano, C.; De Silvestri, A.; Pelizzo, G.; Regalbuto, C.; Paganelli, V.; Albertini, R.; Cave, F.D.; Larizza, D.; Cena, H. Triglyceride Glucose Index as a Surrogate Measure of Insulin Sensitivity in a Caucasian Pediatric Population. J. Clin. Res. Pediatr. Endocrinol. 2020. [Google Scholar] [CrossRef]
- Flegal, K.M.; Cole, T.J. Construction of LMS Parameters for the Centers for Disease Control and Prevention 2000 Growth Charts; National Center for Health Statistics: Hyattsville, MD, USA, 2013; pp. 1–3.
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Eckel, R.H.; Alberti, K.G.M.M.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2010, 375, 181–183. [Google Scholar] [CrossRef]
- VanLancker, T.; Schaubroeck, E.; Vyncke, K.; Cadenas-Sanchez, C.; Breidenassel, C.; González-Gross, M.; Gottrand, F.; Moreno, L.A.; Beghin, L. Comparison of definitions for the metabolic syndrome in adolescents. The HELENA study. Eur. J. Nucl. Med. Mol. Imaging 2017, 176, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Perez-Pozo, S.E.; Sautin, Y.Y.; Manitius, J.; Sanchez-Lozada, L.G.; Feig, D.I.; Shafiu, M.; Segal, M.; Glassock, R.J.; Shimada, M.; et al. Hypothesis: Could Excessive Fructose Intake and Uric Acid Cause Type 2 Diabetes? Endocr. Rev. 2009, 30, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calcaterra, V.; Cena, H.; De Silvestri, A.; Di Mitri, M.; Pelizzo, G. Disorders of Puberty in Severely Neurologically Impaired Children: Is Delayed Puberty an Underestimated Problem? Front. Pediatr. 2019, 7, 462. [Google Scholar] [CrossRef] [Green Version]
- Langhans, W. Peripheral mechanisms involved with catabolism. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 419–426. [Google Scholar] [CrossRef]





| Variables | Children with Disabilities n = 44 | Healthy Control Children n = 120 |
|---|---|---|
| Age * (years) | 14.47 (6.85, 17.62) | 11.01 (8.87, 13.32) |
| Tanner stages (n) | ||
| -Tanner 1 | 15 (34.1%) | 36 (30.0%) |
| -Tanner 2–3 | 23 (52.3%) | 66 (55.0%) |
| -Tanner 4–5 | 6 (13.6%) | 18 (15.0%) |
| Height * (cm) | 135.5 (114.2, 145.2) | 143.9 (135.3, 154.1) |
| Height z-score [32] | −2.01 (−4.02, −0.76) | −0.14 (−0.87, 0.98) |
| Weight * (kg) | 27.55 (17.68, 34.70) | 35.50 (30.00, 46.25) |
| Weight z-score [32] | −0.67 (−1.52, 0.27) | 0.76 (−0.47, 1.37) |
| Body mass index (kg/m2) * | 15.70 (13.82, 18.05) | 17.35 (15.79, 19.53) |
| BMI z-score [32] | −1.36 (−2.95, −0.12) | 0.076 (−0.53, 0.77) |
| Fasting glucose (mg/dL) * | 73.00 (59.75, 90.00) | 75.00 (69.00, 80.00) |
| Fasting insulin (U/mL) * | 14.95 (5.55, 23.07) | 5.300 (3.100, 8.40) |
| HOMA-IR * | 3.01 (1.02, 4.64) | 0.58 (0.31, 1.12) |
| Fasting triglycerides (mg/dL) * | 83.5 (66.0, 106.5) | 46.0 (38.0, 57.0) |
| Total cholesterol (mg/dL) * | 134.5 (110.8, 166.2) | 156.0 (136.0, 170.8) |
| HDL cholesterol (mg/dL) * | 43.00 (37.00, 48.00) | 53.50 (47.00, 60.00) |
| Systolic blood pressure (mmHg) * | 100.5 (90.5, 116.0) | 100.0 (95.0, 110.0) |
| Diastolic blood pressure (mmHg) * | 65.00 (56.00, 76.00) | 60.00 (60.00, 70.00) |
| Trygliceride glucose index * | 8.08 (7.80, 8.35) | 7.44 (7.22, 7.67) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Biganzoli, G.; Pelizzo, G.; Cena, H.; Rizzuto, A.; Penagini, F.; Verduci, E.; Bosetti, A.; Lucini, D.; Biganzoli, E.; et al. A Multivariate Pattern Analysis of Metabolic Profile in Neurologically Impaired Children and Adolescents. Children 2021, 8, 186. https://0-doi-org.brum.beds.ac.uk/10.3390/children8030186
Calcaterra V, Biganzoli G, Pelizzo G, Cena H, Rizzuto A, Penagini F, Verduci E, Bosetti A, Lucini D, Biganzoli E, et al. A Multivariate Pattern Analysis of Metabolic Profile in Neurologically Impaired Children and Adolescents. Children. 2021; 8(3):186. https://0-doi-org.brum.beds.ac.uk/10.3390/children8030186
Chicago/Turabian StyleCalcaterra, Valeria, Giacomo Biganzoli, Gloria Pelizzo, Hellas Cena, Alessandra Rizzuto, Francesca Penagini, Elvira Verduci, Alessandra Bosetti, Daniela Lucini, Elia Biganzoli, and et al. 2021. "A Multivariate Pattern Analysis of Metabolic Profile in Neurologically Impaired Children and Adolescents" Children 8, no. 3: 186. https://0-doi-org.brum.beds.ac.uk/10.3390/children8030186