Specialized Pediatric Palliative Care Services in Pediatric Hematopoietic Stem Cell Transplant Centers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
2.2. Survey
3. Results
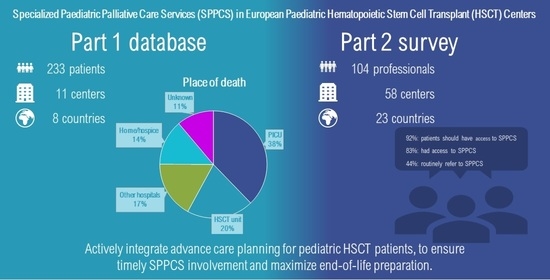
3.1. Database
Place of Death and Cause of Death
3.2. Survey
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Passweg, J.R.; Baldomero, H.; Basak, G.W.; Chabannon, C.; Corbacioglu, S.; Duarte, R.; Kuball, J.; Lankester, A.; Montoto, S.; de Latour, R.P.; et al. The EBMT activity survey report 2017: A focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transplant. 2019. [Google Scholar] [CrossRef] [Green Version]
- Passweg, J.R.; Baldomero, H.; Peters, C.; Gaspar, H.B.; Cesaro, S.; Dreger, P.; Duarte, R.F.; Falkenburg, J.H.; Farge-Bancel, D.; Gennery, A.; et al. Hematopoietic SCT in Europe: Data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplant. 2014, 49, 744–750. [Google Scholar] [CrossRef]
- Di Giuseppe, G.; Thacker, N.; Schechter, T.; Pole, J.D. Anxiety, depression, and mental health-related quality of life in survivors of pediatric allogeneic hematopoietic stem cell transplantation: A systematic review. Bone Marrow Transplant. 2020, 55, 1240–1254. [Google Scholar] [CrossRef]
- Snaman, J.M.; Talleur, A.C.; Lu, J.; Levine, D.R.; Kaye, E.C.; Sykes, A.; Lu, Z.; Triplett, B.M.; Baker, J.N. Treatment intensity and symptom burden in hospitalized adolescent and young adult hematopoietic cell transplant recipients at the end of life. Bone Marrow Transplant. 2018, 53, 84–90. [Google Scholar] [CrossRef]
- El-Jawahri, A.; LeBlanc, T.; VanDusen, H.; Traeger, L.; Greer, J.A.; Pirl, W.F.; Jackson, V.A.; Telles, J.; Rhodes, A.; Spitzer, T.R.; et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2016, 316, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, C.K.; Lehmann, L.; London, W.B.; Guo, D.; Sridharan, M.; Koch, R.; Wolfe, J. End-of-Life Care Patterns Associated with Pediatric Palliative Care among Children Who Underwent Hematopoietic Stem Cell Transplant. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1049–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szmit, Z.; Kośmider-Żurawska, M.; Król, A.; Łobos, M.; Miśkiewicz-Bujna, J.; Zielińska, M.; Kałwak, K.; Mielcarek-Siedziuk, M.; Salamonowicz-Bodzioch, M.; Frączkiewicz, J.; et al. Factors affecting survival in children requiring intensive care after hematopoietic stem cell transplantation. A retrospective single-center study. Pediatric Transplant. 2020, 24, e13765. [Google Scholar] [CrossRef]
- Ullrich, C.K.; Dussel, V.; Hilden, J.M.; Sheaffer, J.W.; Lehmann, L.; Wolfe, J. End-of-life experience of children undergoing stem cell transplantation for malignancy: Parent and provider perspectives and patterns of care. Blood 2010, 115, 3879–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadatou, D.; Kalliani, V.; Karakosta, E.; Liakopoulou, P.; Bluebond-Langner, M. Home or hospital as the place of end-of-life care and death: A grounded theory study of parents’ decision-making. Palliat. Med. 2021, 35, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Feudtner, C.; Feinstein, J.A.; Satchell, M.; Zhao, H.; Kang, T.I. Shifting place of death among children with complex chronic conditions in the United States, 1989-2003. JAMA 2007, 297, 2725–2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Håkanson, C.; Öhlén, J.; Kreicbergs, U.; Cardenas-Turanzas, M.; Wilson, D.M.; Loucka, M.; Frache, S.; Giovannetti, L.; Naylor, W.; Rhee, Y.; et al. Place of death of children with complex chronic conditions: Cross-national study of 11 countries. Eur. J. Pediatrics 2017, 176, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.E.; Martinez, I.; Wolfe, J.; Asch, S.M. Quality measures for end-of-life care for children with cancer: A modified Delphi approach. Cancer 2021, 127, 2571–2578. [Google Scholar] [CrossRef]
- Ananth, P.; Mun, S.; Reffat, N.; Li, R.; Sedghi, T.; Avery, M.; Snaman, J.; Gross, C.P.; Ma, X.; Wolfe, J. A Stakeholder-Driven Qualitative Study to Define High Quality End-of-Life Care for Children with Cancer. J. Pain. Symptom. Manag. 2021. [Google Scholar] [CrossRef]
- Moody, K.M.; Hendricks-Ferguson, V.L.; Baker, R.; Perkins, S.; Haase, J.E. A Pilot Study of the Effects of COMPLETE: A Communication Plan Early Through End of Life, on End-of-Life Outcomes in Children with Cancer. J. Pain. Symptom. Manag. 2020, 60, 417–421. [Google Scholar] [CrossRef]
- World Health Organization. Global Atlas of Palliative Care at the End of Life; WHO: Rome, Italy, 2014; Available online: https://www.who.int/nmh/Global_Atlas_of_Palliative_Care.pdf (accessed on 15 December 2020).
- El-Jawahri, A.; Traeger, L.; Greer, J.A.; VanDusen, H.; Fishman, S.R.; LeBlanc, T.W.; Pirl, W.F.; Jackson, V.A.; Telles, J.; Rhodes, A.; et al. Effect of Inpatient Palliative Care During Hematopoietic Stem-Cell Transplant on Psychological Distress 6 Months after Transplant: Results of a Randomized Clinical Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3714–3721. [Google Scholar] [CrossRef]
- Mekelenkamp, H.; Lankester, A.C.; Bierings, M.B.; Smiers, F.J.W.; de Vries, M.C.; Kars, M.C. Parental experiences in end-of-life decision-making in allogeneic pediatric stem cell transplantation: Have I been a good parent? Pediatric Blood Cancer 2020, e28229. [Google Scholar] [CrossRef] [Green Version]
- Jalmsell, L.; Onelov, E.; Steineck, G.; Henter, J.I.; Kreicbergs, U. Hematopoietic stem cell transplantation in children with cancer and the risk of long-term psychological morbidity in the bereaved parents. Bone Marrow Transplant. 2011, 46, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.; Goodenough, B.; Maurice, L.; Foreman, T.; Willis, L. Parental grieving after a child dies from cancer: Is stress from stem cell transplant a factor? Int. J. Palliat. Nurs. 2005, 11, 266–273. [Google Scholar] [CrossRef]
- van der Geest, I.M.; Darlington, A.S.; Streng, I.C.; Michiels, E.M.; Pieters, R.; van den Heuvel-Eibrink, M.M. Parents’ experiences of pediatric palliative care and the impact on long-term parental grief. J. Pain Symptom Manag. 2014, 47, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.J.; Namisango, E.; Hunt, J.; Powell, R.A.; Baker, J.N. Grief and Bereavement in Parents After the Death of a Child in Low- and Middle-Income Countries. Children 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Loggers, E.T.; Lee, S.; Chilson, K.; Back, A.L.; Block, S.; Loberiza, F.R. Advance care planning among hematopoietic cell transplant patients and bereaved caregivers. Bone Marrow Transplant. 2014, 49, 1317–1322. [Google Scholar] [CrossRef]
- RCoreTeam. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 23 January 2021).
- FACT-JACIE. International Standard for Hematopoietic Cellular Therapy Product Collection, Processing, and Administration. 2018. Available online: https://www.ebmt.org/sites/default/files/2018-06/FACT-JACIE%207th%20Edition%20Standards.pdf (accessed on 28 April 2021).
- Weaver, M.S.; Rosenberg, A.R.; Tager, J.; Wichman, C.S.; Wiener, L. A Summary of Pediatric Palliative Care Team Structure and Services as Reported by Centers Caring for Children with Cancer. J. Palliat. Med. 2018, 21, 452–462. [Google Scholar] [CrossRef]
- El-Jawahri, A.; LeBlanc, T.W.; Burns, L.J.; Denzen, E.; Meyer, C.; Mau, L.W.; Roeland, E.J.; Wood, W.A.; Petersdorf, E. What Do Transplant Physicians Think About Palliative Care? A National Survey Study. Cancer 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haines, E.R.; Frost, A.C.; Kane, H.L.; Rokoske, F.S. Barriers to accessing palliative care for pediatric patients with cancer: A review of the literature. Cancer 2018, 124, 2278–2288. [Google Scholar] [CrossRef] [Green Version]
- Booker, R.; Simon, J.; Biondo, P.; Bouchal, S.R. Perspectives on advance care planning in haematopoietic stem cell transplantation: A qualitative study. Int. J. Palliat. Nurs. 2018, 24, 132–144. [Google Scholar] [CrossRef]
- Fahner, J.C.; Beunders, A.J.M.; van der Heide, A.; Rietjens, J.A.C.; Vanderschuren, M.M.; van Delden, J.J.M.; Kars, M.C. Interventions Guiding Advance Care Planning Conversations: A Systematic Review. J. Am. Med. Dir. Assoc. 2019, 20, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Fahner, J.C.; Tholking, T.W.; Rietjens, J.A.C.; van der Heide, A.; van Delden, J.J.M.; Kars, M.C. Towards advance care planning in pediatrics: A qualitative study on envisioning the future as parents of a seriously ill child. Eur. J. Pediatrics 2020. [Google Scholar] [CrossRef] [Green Version]
- Levine, D.R.; Baker, J.N.; Wolfe, J.; Lehmann, L.E.; Ullrich, C. Strange Bedfellows No More: How Integrated Stem-Cell Transplantation and Palliative Care Programs Can Together Improve End-of-Life Care. J. Oncol. Pract. 2017, 13, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Dains, J.E. Advanced Care Planning and End-of-life Outcomes in Hematopoietic Stem Cell Transplant Patients. Am. J. Hosp. Palliat. Care 2021, 38, 995–1003. [Google Scholar] [CrossRef]
- Mitchell, S.A. Palliative care during and following allogeneic hematopoietic stem cell transplantation. Curr. Opin. Support. Palliat. Care 2018, 12, 58–64. [Google Scholar] [CrossRef]
- Lafond, D.A.; Kelly, K.P.; Hinds, P.S.; Sill, A.; Michael, M. Establishing feasibility of early palliative care consultation in pediatric hematopoietic stem cell transplantation. J. Pediatric Oncol. Nurs. 2015, 32, 265–277. [Google Scholar] [CrossRef] [PubMed]


| Variables | Modalities | All Patients (n = 233) | At Home or Hospice (n = 32) | PICU (n = 88) | Other Hospital (n = 40) | HSCT Unit (n = 47) | Unknown Place of Death by the Center (n = 26) | Test p-Value |
|---|---|---|---|---|---|---|---|---|
| Age at first HSCT | median [IQR] | 6.01 [2.6–11.8] | 7.9 [3.1–10.7] | 5.7 [1.7–12.8] | 4.8 [2.1–11.8] | 6.9 [2.3–10.8] | 7 [3.5–11.6] | 0.6 |
| (range) | (0.2–18) | (1.1–17.8) | (0.2–18) | (0.2–17.4) | (0.4–15.9) | (0.6–17.9) | ||
| Patient sex n (%) | Female | 94 (40.3) | 15 (16.0) | 37 (39.4) | 10 (10.6) | 18 (19.1) | 14 (14.9) | 0.16 |
| Male | 139 (59.7) | 17 (12.2) | 51 (36.7) | 30 (21.6) | 29 (20.9) | 12 (8.6) | ||
| Time between last HSCT and death (in month) | median [IQR] | 3.8 [1.6–9.1] | 8.7 [4.1–17.4] | 1.8 [0.8–4.4] | 4.8 [3.6–11.4] | 2.6 [1.4–4.4] | 8.2 [3.8–17.7] | <0.0001 |
| (range) | (0–48.9) | (1.6–48.9) | (0.1–37.1) | (0.6–47.5) | (0–28.8) | (1.8–36.8) | ||
| Age at death (in years) | median [IQR] | 7.2 [3.3–13.2] | 8.9 [4.9–13.1] | 6.9 [2.6–13.2] | 6.5 [3.6–12.6] | 7.4 [2.5–12.3] | 8.7 [4.2–12.7] | 0.48 |
| (range) | (0.3–21.6) | (1.8–20.2) | (0.3–21.6) | (0.6–19) | (0.6–17.9) | (2.5–19.5) | ||
| Cause of death * n (%) | HSCT-related | 137 (59.3) | 5 (3.6) | 76 (55.5) | 15 (10.9) | 34 (24.8) | 7 (5.1) | <0.0001 |
| Disease-related | 94 (40.7) | 27 (28.7) | 12 (12.8) | 24 (25.5) | 13 (13.8) | 18 (19.1) | ||
| missing | 2 | 0 | 0 | 1 | 0 | 1 | ||
| Total number of HSCT n (%) | Single HSCT | 176 (75.5) | 28 (15.9) | 68 (38.6) | 31 (17.6) | 29 (16.5) | 20 (11.4) | 0.11 |
| Multiple HSCT | 57 (24.5) | 4 (7.0) | 20 (35.1) | 9 (15.8) | 18 (31.6) | 6 (10.5) | ||
| Disease ** n (%) | Malignant | 147 (63.1) | 29 (19.7) | 47 (32.0) | 26 (17.7) | 21 (14.3) | 24 (16.3) | <0.0001 |
| Non-Malignant | 86 (36.9) | 3 (3.5) | 41 (47.7) | 14 (16.3) | 26 (30.2) | 2 (2.3) |
| Variables | Modalities | All Patients (n = 233) | Malignant (n = 147) | Non-Malignant (n = 86) | Test p-Value |
|---|---|---|---|---|---|
| Age at first HSCT | median [IQR] | 6.0 [2.6–11.8] | 7.5 [4–13] | 2.7 [0.7–8.4] | <0.0001 |
| (range) | (0.2–18) | (0.5–17.9) | (0.2–18) | ||
| Patient sex n (%) | Female | 94 (40.3) | 59 (40.1) | 35 (40.7) | 0.93 |
| Male | 139 (59.7) | 88 (59.9) | 51 (59.3) | ||
| Time between last HSCT and death (month) | median [IQR] | 3.8 [1.6–9.1] | 4.8 [2.7–10.9] | 2.1 [1.1–4.2] | <0.0001 |
| (range) | (0–48.9) | (0.1–48.9) | (0–47.5) | ||
| Age at death | median [IQR] | 7.2 [3.3–13.2] | 8.9 [5.4–14.3] | 3.5 [1.4–9.2] | <0.0001 |
| (range) | (0.3–21.6) | (0.7–21.6) | (0.3–18.1) | ||
| Cause of death | HSCT-related | 137 (59.3) | 65 (44.5) | 72 (84.7) | <0.0001 |
| n (%) | Disease-related | 94 (40.7) | 81 (55.5) | 13 (15.3) | |
| missing | 2 | 1 | 1 | ||
| Total number of HSCT | Single HSCT | 176 (75.5) | 109 (74.1) | 67 (77.9) | 0.52 |
| n (%) | Multiple HSCT | 57 (24.5) | 38 (25.9) | 19 (22.1) | |
| Total number of HSCT (detailed) | 1 | 176 (75.5) | 109 (74.1) | 67 (77.9) | Not tested |
| n (%) | 2 | 45 (19.3) | 32 (21.8) | 13 (15.1) | |
| 3 | 9 (3.9) | 6 (4.1) | 3 (3.5) | ||
| 4 | 3 (1.3) | 0 (0) | 3 (3.5) |
| Questions | Responses | All Respondents (n = 98) | Physician (n = 42) | Nurse (n = 40) | Affiliated (n = 16) |
|---|---|---|---|---|---|
| Years working in current function n (%) | 0 to 5 | 19 (21.6) | 7 (20) | 7 (18.9) | 5 (31.2) |
| 5 to 10 | 14 (15.9) | 7 (20) | 4 (10.8) | 3 (18.8) | |
| 10 to 15 | 15 (17) | 4 (11.4) | 6 (16.2) | 5 (31.2) | |
| 15–20 | 14 (15.9) | 5 (14.3) | 7 (18.9) | 2 (12.5) | |
| >20 | 26 (29.5) | 12 (34.3) | 13 (35.1) | 1 (6.2) | |
| missing | 10 | 7 | 3 | 0 | |
| Age (in years) n (%) | 25–35 | 16 (16.8) | 4 (9.5) | 6 (16.2) | 6 (37.5) |
| 35–40 | 12 (12.6) | 7 (16.7) | 4 (10.8) | 1 (6.2) | |
| 40–55 | 50 (52.6) | 20 (47.6) | 22 (59.5) | 8 (50) | |
| 55–60 | 11 (11.6) | 6 (14.3) | 4 (10.8) | 1 (6.2) | |
| >60 | 6 (6.3) | 5 (11.9) | 1 (2.7) | 0 (0) | |
| missing | 3 | 0 | 3 | 0 | |
| Sex n (%) | Female | 78 (79.6) | 26 (61.9) | 37 (92.5) | 15 (93.8) |
| Male | 20 (20.4) | 16 (38.1) | 3 (7.5) | 1 (6.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekelenkamp, H.; Schröder, T.; Trigoso, E.; Hutt, D.; Galimard, J.-E.; Kozijn, A.; Dalissier, A.; Gjergji, M.; Liptrott, S.; Kenyon, M.; et al. Specialized Pediatric Palliative Care Services in Pediatric Hematopoietic Stem Cell Transplant Centers. Children 2021, 8, 615. https://0-doi-org.brum.beds.ac.uk/10.3390/children8080615
Mekelenkamp H, Schröder T, Trigoso E, Hutt D, Galimard J-E, Kozijn A, Dalissier A, Gjergji M, Liptrott S, Kenyon M, et al. Specialized Pediatric Palliative Care Services in Pediatric Hematopoietic Stem Cell Transplant Centers. Children. 2021; 8(8):615. https://0-doi-org.brum.beds.ac.uk/10.3390/children8080615
Chicago/Turabian StyleMekelenkamp, Hilda, Teija Schröder, Eugenia Trigoso, Daphna Hutt, Jacques-Emmanuel Galimard, Anne Kozijn, Arnaud Dalissier, Marjola Gjergji, Sarah Liptrott, Michelle Kenyon, and et al. 2021. "Specialized Pediatric Palliative Care Services in Pediatric Hematopoietic Stem Cell Transplant Centers" Children 8, no. 8: 615. https://0-doi-org.brum.beds.ac.uk/10.3390/children8080615