Increased Proliferation of Neuroblastoma Cells under Fructose Metabolism Can Be Measured by Isothermal Microcalorimetry
Abstract
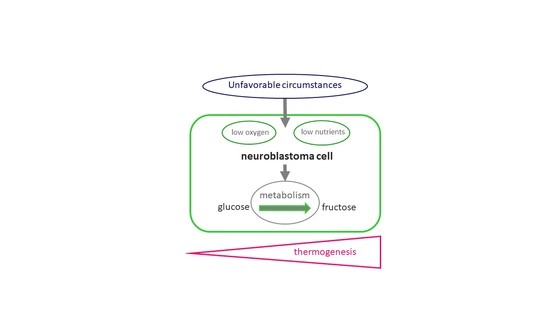
:1. Introduction
2. Materials and Methods
2.1. Cell Proliferation
2.2. Isothermal Microcalorimetry
3. Results
4. Discussion
4.1. Thermogenesis
4.2. Tumor Cell Metabolism
4.3. Tumor Cells Proliferation and Migration
4.4. Clinical Significance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Modak, S.; Cheung, N.K. Neuroblastoma: Therapeutic strategies for a clinical enigma. Cancer Treat. Rev. 2010, 36, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.K.; Dyer, M.A. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [Green Version]
- Louis, C.U.; Shohet, J.M. Neuroblastoma: Molecular pathogenesis and therapy. Annu. Rev. Med. 2015, 66, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, Z.; Sa Santos, M.; Drenckhan, A.; Holland-Cunz, S.; Izbicki, J.R.; Nash, M.A.; Gros, S.J. Metastatic Esophageal Carcinoma Cells Exhibit Reduced Adhesion Strength and Enhanced Thermogenesis. Cells 2021, 10, 1213. [Google Scholar] [CrossRef]
- Gros, S.J.; Holland-Cunz, S.G.; Supuran, C.T.; Braissant, O. Personalized Treatment Response Assessment for Rare Childhood Tumors Using Microcalorimetry-Exemplified by Use of Carbonic Anhydrase IX and Aquaporin 1 Inhibitors. Int. J. Mol. Sci. 2019, 20, 4984. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. Iron, the Oxygen-Carrier of Respiration-Ferment. Science 1925, 61, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Lanaspa, M.A.; Millan, I.S.; Fini, M.; Rivard, C.J.; Sanchez-Lozada, L.G.; Andres-Hernando, A.; Tolan, D.R.; Johnson, R.J. Fructose contributes to the Warburg effect for cancer growth. Cancer Metab. 2020, 8, 16. [Google Scholar] [CrossRef]
- Wadso, I. Isothermal microcalorimetry in applied biology. Thermochim. Acta 2002, 394, 305–311. [Google Scholar] [CrossRef]
- Lemos, D.; Oliveira, T.; Martins, L.; de Azevedo, V.R.; Rodrigues, M.F.; Ketzer, L.A.; Rumjanek, F.D. Isothermal Microcalorimetry of Tumor Cells: Enhanced Thermogenesis by Metastatic Cells. Front. Oncol. 2019, 9, 1430. [Google Scholar] [CrossRef]
- Lutz, W.; Fulda, S.; Jeremias, I.; Debatin, K.M.; Schwab, M. MycN and IFNgamma cooperate in apoptosis of human neuroblastoma cells. Oncogene 1998, 17, 339–346. [Google Scholar] [CrossRef] [Green Version]
- Lutz, W.; Stohr, M.; Schurmann, J.; Wenzel, A.; Lohr, A.; Schwab, M. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene 1996, 13, 803–812. [Google Scholar]
- Braissant, O.; Keiser, J.; Meister, I.; Bachmann, A.; Wirz, D.; Gopfert, B.; Bonkat, G.; Wadso, I. Isothermal microcalorimetry accurately detects bacteria, tumorous microtissues, and parasitic worms in a label-free well-plate assay. Biotechnol. J. 2015, 10, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Braissant, O.; Muller, G.; Egli, A.; Widmer, A.; Frei, R.; Halla, A.; Wirz, D.; Gasser, T.C.; Bachmann, A.; Wagenlehner, F.; et al. Seven hours to adequate antimicrobial therapy in urosepsis using isothermal microcalorimetry. J. Clin. Microbiol. 2014, 52, 624–626. [Google Scholar] [CrossRef] [Green Version]
- Galindo, F.G.; Rocculi, P.; Wadso, L.; Sjohlm, I. The potential of isothermal calorimetry in monitoring and predicting quality changes during processing and storage of minimally processed fruits and vegetables. Trends Food Sci. Tech. 2005, 16, 325–331. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Fathi, M.H.; Karbasi, S.; Braissant, O.; Daniels, A.U. Direct cytotoxicity evaluation of 63S bioactive glass and bone-derived hydroxyapatite particles using yeast model and human chondrocyte cells by microcalorimetry. J. Mater. Sci. Mater. Med. 2011, 22, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Hosios, A.M.; Hecht, V.C.; Danai, L.V.; Johnson, M.O.; Rathmell, J.C.; Steinhauser, M.L.; Manalis, S.R.; Vander Heiden, M.G. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev. Cell 2016, 36, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locasale, J.W.; Grassian, A.R.; Melman, T.; Lyssiotis, C.A.; Mattaini, K.R.; Bass, A.J.; Heffron, G.; Metallo, C.M.; Muranen, T.; Sharfi, H.; et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 2011, 43, 869–874. [Google Scholar] [CrossRef] [Green Version]
- Lassen, U.; Daugaard, G.; Eigtved, A.; Damgaard, K.; Friberg, L. 18F-FDG whole body positron emission tomography (PET) in patients with unknown primary tumours (UPT). Eur. J. Cancer 1999, 35, 1076–1082. [Google Scholar] [CrossRef]
- Godoy, A.; Ulloa, V.; Rodriguez, F.; Reinicke, K.; Yanez, A.J.; Garcia Mde, L.; Medina, R.A.; Carrasco, M.; Barberis, S.; Castro, T.; et al. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: Ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J. Cell Physiol. 2006, 207, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xing, S.N.; Tang, H.Y.; Wang, C.D.; Yi, F.P.; Liu, G.L.; Wu, X.M. Influence of glucose transporter 1 activity inhibition on neuroblastoma in vitro. Gene 2019, 689, 11–17. [Google Scholar] [CrossRef]
- Matsushita, K.; Uchida, K.; Saigusa, S.; Ide, S.; Hashimoto, K.; Koike, Y.; Otake, K.; Inoue, M.; Tanaka, K.; Kusunoki, M. Glycolysis inhibitors as a potential therapeutic option to treat aggressive neuroblastoma expressing GLUT1. J. Pediatric. Surg. 2012, 47, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Mahraoui, L.; Rodolosse, A.; Barbat, A.; Dussaulx, E.; Zweibaum, A.; Rousset, M.; Brot-Laroche, E. Presence and differential expression of SGLT1, GLUT1, GLUT2, GLUT3 and GLUT5 hexose-transporter mRNAs in Caco-2 cell clones in relation to cell growth and glucose consumption. Biochem. J. 1994, 298 Pt 3, 629–633. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Leon, S.P.; Golde, D.W.; Concha, I.I.; Rivas, C.I.; Delgado-Lopez, F.; Baselga, J.; Nualart, F.; Vera, J.C. Expression of the fructose transporter GLUT5 in human breast cancer. Proc. Natl. Acad. Sci. USA 1996, 93, 1847–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Johnson, R.J.; Andres-Hernando, A.; Roncal-Jimenez, C.; Sanchez-Lozada, L.G.; Tolan, D.R.; Lanaspa, M.A. Fructose Production and Metabolism in the Kidney. J. Am. Soc. Nephrol. JASN 2020, 31, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Zhu, J.; Chen, Z.; Fu, J.; Zhang, F. Fructose fuels lung adenocarcinoma through GLUT5. Cell Death Dis. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Li, N.; Li, Z.; Xu, J.; Su, C. Ketohexokinase is involved in fructose utilization and promotes tumor progression in glioma. Biochem. Biophys. Res. Commun. 2018, 503, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar]
- Mirtschink, P.; Krishnan, J.; Grimm, F.; Sarre, A.; Horl, M.; Kayikci, M.; Fankhauser, N.; Christinat, Y.; Cortijo, C.; Feehan, O.; et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 2015, 522, 444–449. [Google Scholar] [CrossRef]
- Monzavi-Karbassi, B.; Hine, R.J.; Stanley, J.S.; Ramani, V.P.; Carcel-Trullols, J.; Whitehead, T.L.; Kelly, T.; Siegel, E.R.; Artaud, C.; Shaaf, S.; et al. Fructose as a carbon source induces an aggressive phenotype in MDA-MB-468 breast tumor cells. Int. J. Oncol. 2010, 37, 615–622. [Google Scholar] [CrossRef] [Green Version]
- Monzavi-Karbassi, B.; Whitehead, T.L.; Jousheghany, F.; Artaud, C.; Hennings, L.; Shaaf, S.; Slaughter, A.; Korourian, S.; Kelly, T.; Blaszczyk-Thurin, M.; et al. Deficiency in surface expression of E-selectin ligand promotes lung colonization in a mouse model of breast cancer. Int. J. Cancer J. Int. Du Cancer 2005, 117, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Lee, I.; Kamar, M.; Akiyama, S.K.; Pierce, M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res. 2002, 62, 6837–6845. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pini, N.; Huo, Z.; Holland-Cunz, S.; Gros, S.J. Increased Proliferation of Neuroblastoma Cells under Fructose Metabolism Can Be Measured by Isothermal Microcalorimetry. Children 2021, 8, 784. https://0-doi-org.brum.beds.ac.uk/10.3390/children8090784
Pini N, Huo Z, Holland-Cunz S, Gros SJ. Increased Proliferation of Neuroblastoma Cells under Fructose Metabolism Can Be Measured by Isothermal Microcalorimetry. Children. 2021; 8(9):784. https://0-doi-org.brum.beds.ac.uk/10.3390/children8090784
Chicago/Turabian StylePini, Nicola, Zihe Huo, Stefan Holland-Cunz, and Stephanie J. Gros. 2021. "Increased Proliferation of Neuroblastoma Cells under Fructose Metabolism Can Be Measured by Isothermal Microcalorimetry" Children 8, no. 9: 784. https://0-doi-org.brum.beds.ac.uk/10.3390/children8090784