1. Introduction
Biological systems, by nature, are highly complex. The biosystems’ emerging patterns are far more complicated than any other soft system. These features are hard to predict and susceptible, since many biological activities are interconnected and largely depend on vast parameter space. The mesoscopic or macroscopic resulting patterns are primarily sensitive to the initial conditions, including the preparation of the samples, perturbing fields, and so on [
1]. A systematic and consistent approach is recommended to explore these systems that require altering one parameter at a time. The complex patterns associated with these bio-systems are due to the local self-assembling interactions between the constituent particles [
2]. The transportation of a system from one state (initial bio-colloidal fluid) to another (dried film) through a non-equilibrium (drying) process requires (i) exchanging energy and matter to get acquainted with its micro-environment, and (ii) adjusting the self-assembling interactions between the constituent particles [
3].
The research findings of the drying drop community have exhibited numerous studies in the salt-colloidal systems [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. However, the number of findings drops off when the biological systems in the presence of external salts are concerned. Two globular protein samples, lysozyme and bovine serum albumin (BSA), are predominantly investigated to understand how the patterns are affected when the initial salt concentration is varied [
15,
16,
17,
18,
19,
20,
21,
22]. Gorr et al. [
18] examined lysozyme protein at various concentrations of NaCl. The study by Gorr et al. reported the presence of three distinct regions in the drops with NaCl. The first one is formed in the peripheral ring, where most lysozyme is present. The second one forms different salt structures that occupy the secondary ring area (observed adjacent to the ring), and the final one is observed in the central regions. A similar observation is also reported in BSA-saline protein drying drops by Yakhno [
15]. The study by Yakhno concludes that the salt crystals are phase-separated by forming different zones from homogeneous protein film near the periphery to the salt crystals in the central region. Pathak et al. [
23] investigated the effects of multiple salts (MgCl
and KCl) on the BSA patterns. This study reveals that the crystal structures depend on the initial tuning ratio of these salts. Furthermore, a few recent studies examined the formation of these protein-saline drying drops at different elevated substrate temperatures. The findings of these experiments confirmed that the final morphological patterns are mainly dependent on the environmental conditions (higher vs. lower drying rate) [
24,
25,
26].
New and more sophisticated image processing techniques are developed with the increasing demands of examining the complex images of drying stages. Pattern recognition tools, such as k-means clustering and the k-nearest neighbor algorithm, were applied by Gorr et al. [
27] to differentiate the lysozyme-NaCl deposits based on the salts’ initial concentration. Carreón et al. [
21] applied the first-order statistics (FOS), and the gray level co-occurrence matrix (GLCM) specifying the textural image properties. The study explored the evolution of the final state of drying BSA-lysozyme films in NaCl salts’ presence using the FOS and GLCM techniques. Pal et al. [
28] also examined BSA drops at different initial concentrations of phosphate buffer saline (PBS). The statistical analysis incorporated in that work showed that these GLCM parameters’ horizontal and vertical orientations have a non-significant effect when the pixel displacement is ≤1. The pixel distribution is explored at different PBS initial conditions and regions, such as rim and non-rim regions. The study concluded that the BSA–BSA interactions are dominant over the BSA-saline interactions in the rim regions and vice versa.
On the other hand, many researchers also initiated simplification of these systems by reducing the system’s complexity and preparing these protein samples in de-ionized water (without adding any external salts) [
17,
22,
29]. In recent years, Pal et al. provided a new perspective while examining the protein droplets and explored the physics of the drying drops containing optically active particles such as thermotropic liquid crystals [
30,
31,
32]. The complexity of the multi-component system studied by Pal et al. is more or less similar to the protein-saline drops, since both protein and liquid crystals are non-volatile. The detailed findings along these lines imply that the morphological patterns are altered during the drying process when a fixed volume of liquid crystals is added to different globular proteins (lysozyme, BSA, and myoglobin) [
32]. The liquid crystals are distributed randomly in the light-weighted proteins (myoglobin and lysozyme). In contrast, these liquid crystals form umbilical defect structures in heavily weighted proteins such as BSA.
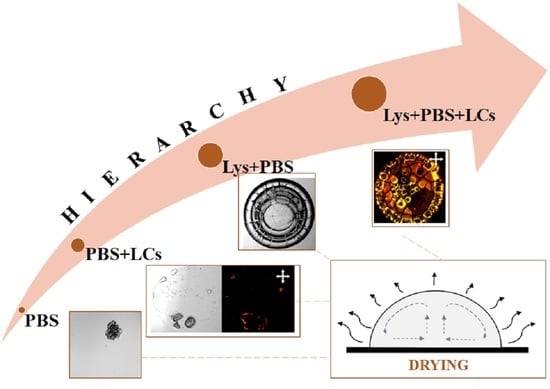
Despite the intense research on protein-saline drying drops, to the best of our knowledge, no systematic study is being performed to understand multiple salts’ effects on various concentrated lysozyme protein solutions. It would also be interesting to extend the work from protein+liquid crystals+water to protein+liquid crystals+PBS. Since the liquid crystals (LCs) have polar head groups, and these salts have multiple ionic charges, it would not be surprising to get complex morphological patterns. However, the question here is what is the best possible way to explore the physics of such a complex system? The current paper systematically examines the drying drop consisting of (i) PBS, (ii) LCs+PBS, (iii) lysozyme+PBS, (iv) lysozyme+PBS+LCs, at different initial concentrations. High-resolution (bright-field, cross-field, and scanning electron) microscopy, image-processing tools, and statistical methods are incorporated in our experiments and analysis to explore their drying evolution and the morphological patterns. In hindsight, this paper attempts to address a few fundamental questions: (i) what are the effects of multiple salts on lysozyme drops with and without LCs? (ii) Do the three regions (as reported in [
18]) in lysozyme-saline drops emerge at every initial concentration? (iii) Does it behave uniformly as reported in the BSA drops? (iv) Is it possible to draw fundamental insights on the self-assembly of LCs, proteins, and salts using the proposed image analysis techniques? If so, how important could these insights be?
2. Materials and Experimental Methods
The materials used in this study include a lyophilized form of hen-egg white lysozyme, PBS (phosphate buffer saline), and thermotropic liquid crystal (5CB (4-Cyano-4-pentyl biphenyl)). The lysozyme (Catalog number L6876) was purchased from Sigma-Aldrich, USA. The different concentrations of the PBS were prepared by diluting 1 (purchased from the Fisher BioReagents, USA (Catalog number BP24384)) into , , and .
The lysozyme has a roughly ellipsoid shape. Its dimension is
nm
, with an aspect ratio of 1.5. Its molecular mass is
kDa [
22]. Lysozyme is made up of 129 amino acids. The isoelectric point of lysozyme is 11.1, which allows it to carry a net positive charge. The globular shape and stability of this protein are attributed to the disulfide bridges, hydrogen bonds, and hydrophobic interactions [
22]. The
PBS solution contains
M (
mg mL
) NaCl, 0.002 M (
mg mL
) KCl, and
M (
mg mL
of Na
HPO
and
mg mL
of KH
PO
) phosphates at a pH of
. The presence of the -cyno groups in 5CB makes it an optically active and polar thermotropic LC. This LC is
nm long and
nm in width, with an aspect ratio of 4. It undergoes a phase transition from a crystalline to a nematic phase at
C and from the nematic LC phase to an isotropic phase at
C [
3].
The various amounts, i.e., 100, 75, 50, 35, 25, and 10 mg of lysozyme, are weighed and mixed separately in 1 mL of these PBS solutions. The samples contained the initial concentrations of lysozyme (, and wt%) and the initial concentrations of the PBS (, and ). The means the de-ionized water (Millipore, 18.2 Mcm at C). Finally, LC (Catalog number 328510, Sigma Aldrich, St. Louis, MO, USA), was heated above its transition temperature, and L was added at the fixed lysozyme concentration ( wt%) as a third component to the different protein-saline drops.
A volume of L sample solution is pipetted on a freshly cleaned coverslip (Catalog number 48366-045, VWR, Radnor, PA, USA) under ambient conditions (the room temperature of C and the relative humidity of %). The drying evolution is monitored every two seconds only for those samples, where the initial lysozyme concentration is kept fixed ( wt%), and the initial PBS concentrations () are varied from 1 to with and without LC droplets. The clock started when the drops were deposited on the substrates. This paper displays the time as , where the total drying time is denoted by , and t is the instantaneous time at which the respective images are captured. The images were captured under magnification using bright-field and cross-polarized optical microscopy (Leitz, Wetzlar, Germany) configured in the transmission mode. An 8-bit digital camera (Model number MU300, Amscope, Irvine, CA, USA) was attached to the microscope to click the top-view images. All these experiments were repeated three times, and all the samples showed the highest reproducibility.
The textural image analysis is performed on the protein drops with and without LCs during the drying process. The oval tool of ImageJ is selected to capture the area of interest in such drops. The first-order statistical image parameters, such as mean gray values (I) and the standard deviation (SD), are extracted using ImageJ [
33]. A detailed discussion on the image analysis process is available in [
26]. A non-parametric Kruskal–Wallis test (an alternative to the parametric one-way ANOVA test) was preferred to examine possible significant interactions among the different initial PBS concentrations (
) in terms of these textural parameters (mean and SD). A similar procedure is also adopted for the samples containing LCs. In the Kruskal–Wallis test, the
is the independent factor, with four levels (groups), 0.25, 0.5, 0.75 and
, whereas the mean and SD are the dependent variables. The significant level is kept as
. A pair-wise comparison between all the
is drawn using the Bonferroni test in R (Version 3.6.3) embedded with R studio (Version 1.2.1335, RStudio, Inc., Boston, MA, USA). For this, the function
pairwise.wilcox.test() with the
p.adjust.method = “bonferroni" is used. The
ggplot2 library and the function
geom_violin() are used to draw the violin plots.
Scanning electron microscopy (JEOL-7000F, JEOL Inc., Peabody, MA, USA) is used for the samples by varying of , and wt% at a fixed of and wt%, ). For this, the nm layer of gold nanoparticles is sputter-coated, and the images are captured at the accelerating voltage of 3 kV and the probe current of 5 mA.
4. Discussions
The height and the contact angle start reducing as soon as the drops are pipetted on the substrate. The non-uniform textural gradient in the optical images (for example,
Figure 5A,B) indicates that the height of the drop initiates the decreasing process in the initial drying stage itself. These drops are of a spherical-cap shape and are partially wet (checked with a goniometer). The curvature of these drops induces the highest mass loss near the periphery compared to their central region. The drops get pinned to the substrate, and the lysozyme particles are transported through the outward capillary radial flow to compensate for this loss. The process leads to the popular “coffee-ring effect” [
34] that is also observed in other bio-colloids as well [
20,
22]. Furthermore, most LC droplets are carried away towards the periphery of the lysozyme drops.
With the progression of the evolution time, the fluid front recedes from the periphery to the central region in the lysozyme drops. Concurrently, the contact angle reduces, contrary to the results reported in [
19]. The deposits in the crack lines (see
Figure 4) indicate a discontinuity in the crack lines at the ring interface (also evident in
Figure 5B,C). It is to be noted that the evaporation of a significant amount of water initiates the formation of salt crystals at this time. Since the images were taken in the transmission mode, the thick film gives a dark texture. The dark textured front starts engulfing the central region (a similar phase transition phenomenon reported in [
15]). A significant fluctuation in the textural evolution (see
Figure 6I–IV) is also observed at this phase. The complexity (SD) increases as the salt crystals (inhomogeneities) appear.
The crack propagation of the pinned drops relieves the mechanical stress. The appearance of the salt crystals in different lysozyme concentrations affects the crack formation process (see
Figure 3). It also alters the interaction between the lysozyme particles and changes their aggregation and precipitation processes (samples with and without adding external salts, evident from
Figure 3 and
Figure 4). The images captured at high and low initial concentrations of PBS (
) at (i) fixed and (ii) different initial concentrations of lysozyme (
) reveal contrasting morphological characteristics. For example, the dried drops formed at
= 1 wt%, (
) = 0.25×, exhibit a rough texture with block salt crystals in the central region. On the other hand, a very thin “coffee ring” and small needle-shaped structures appear in the peripheral region (see
Figure 3 and
Figure 4(I,e)). In contrast, deposits with higher
contain a thick “coffee ring” near the edge, and large rosette-like crystal structures appear in the central region (see
Figure 4(IV,c)). These contrasting morphologies emerge from the different nucleation and super-saturation points during the drying process. Interestingly, this appearance of different structural morphology is not limited to the protein drops but is also reported to be observed in many polymer-saline drops [
14].
This study also demonstrates that the three prominent regions (as reported in [
18]) may or may not surf in the lysozyme drops when the salts are in the solution. This study, therefore, argues that the occurrence of three distinct regions should not be generalized for lysozyme+PBS drying drops. We further argue that the three distinct regions in the lysozyme drops depend on the relative initial concentrations of both lysozyme and salts. The only variation of the salt content might not provide us with a clear picture.
Figure 3 and
Figure 4 show that the chemistry between multiple salts and lysozyme at various initial concentrations (both lysozyme and salts) is the crucial factor in determining morphological patterns. In this context, we established a phase diagram based on the initial concentrations of lysozyme (
) and PBS (
).
Figure 11 exhibits the various phases that are colored uniquely. For example, the orange color describes a phase where the drops always show one ring that divides the central region from the periphery. Two representative images are shown in this phase to illustrate that the central region’s grainy texture changes to black as we increase the
. However, we are ignoring this gradual textural change and counting all in one phase. Though the phase of white color also shows one ring, we used a different color to indicate that the texture of the central regions of both the phases is not the same. The phase of blue color suggests that the drops have multiple rings in the central region in addition to the peripheral ring (illustrated with the dashed red colored line in
Figure 11). The green color indicates the presence of only two rings (one in the central region and another in the peripheral ring). The hatched lines for
indicate that the drops without adding any external salts are totally different from those with PBS. The presence of a mound-like structure (illustrated with the solid red colored line in
Figure 11) in the central region confirms that the underlying physics of such drying-mediated patterns are different for the drops with and without salts. Different phases in
Figure 11 also confirms that the hierarchical structures that are formed by the aggregation of the lysozyme and salts are not directly correlated with their initial concentrations. If so, we would have the same phase as we double their concentrations. For instance, that phase at (
,
) = (
wt%,
) is not the same as the (
,
) = (
wt%,
) or (
,
) = (
wt%,
) is not the same phase as (
,
) = (
wt%,
).
However, it is hard to predict how the adsorption process of various particles (lysozyme and salts) occurs during the drying process. The SEM images of lysozyme+PBS drops (see
Figure 4I–IV) indicate that the lysozyme forms a film on the substrate during the initial drying stage. The salts are phase-separated during the mid-drying stage, forming two different regions. The peripheral region contains most lysozyme particles, whereas the central region contains lysozyme and salts. It is worthy of mentioning here that salt crystallization is a natural process formed due to the evaporation of a large volume of water from the system (similar to the salt formation in the sea beds). It can be assumed that the lysozyme particles in the film act as the nucleation points and initiate distinct types of salt crystallization. For instance, some salts crystallize to form dendrite-like structures on the protein films, whereas some appear as snowflakes, sword-like structures, etc. Some aggregated globules are also present in the crack lines (see
Figure 12(AI,AII)). In this context, a plausible mechanism can be drawn in terms of the protein charges, affinity of salts, surface properties, etc. It can be assumed that the globule nature of these proteins is maintained. As a result, the hydrophobic residues of the proteins are buried inside the protein core, and both positively and negatively charged residues on the protein surface are exposed. It is to be noted that the coverslip (substrate) is negatively charged, and the overall charge of lysozyme is positive. Thus, the positively charged residues adsorb the substrate [
22]. The probability of altering the overall interaction of lysozyme with the substrate is very low.
On the other hand, there is a high chance of different lysozyme–lysozyme and/or lysozyme–salts interactions. This is because the interactions between opposing surface charges and hydrophobic regions binding these globular proteins might be favored. Similarly, the interaction between the lysozyme particles and the solvent produces a charged layer (Stern layer of counter-ions) [
37]. This layer comprises water molecules and/or different dissociating ions present in PBS (viz., Na
, K
, Cl
). The oxygen and hydrogen atoms present in the water are likely to be attached to the protein surfaces’ positively and negatively charged ions, respectively, due to their electrostatic interactions. The further progression of the drying process leads to the modification of the proteins’ hydration shell. Finally, the evaporation of a significant amount of water from the drops facilitates the emergence of the super-saturation or nucleation points.
The addition of LC droplets in the lysozyme drops makes the scenario far more complicated. Let us look at the drops prepared in the de-ionized water (
). The LCs fill the crack lines of the drop at random. Once the protein-cracked domains buckled up due to mechanical stress (see
Figure 3,
wt%,
)), the randomly distributed LCs are sucked underneath these domains (see
Figure 12(BIII,BV)). Accordingly, we can confirm that the bright regions observed under crossed polarized configurations are the randomly oriented LCs distributed underneath each lysozyme domain. The dark region in each domain corresponds to the attached protein layer that is not optically active. A detailed discussion on this is available in [
30]. It is to be noted that the aspect ratios of lysozyme and LC are 1.5 and 4, respectively. Therefore, it can be assumed that there is a probability of a size effect on the crack formation as reported in other multi-component systems [
22,
38]. The bright-field images of the lysozyme drops with and without LCs show that the cracks are more ordered in the presence of LCs, whereas a chaotic system is observed in their absence [
31]. However, extracting the exact physical mechanism due to the difference in their sizes is not within the scope of this paper.
A proper explanation of the drying mechanism in the presence of salts, lysozyme, and LC droplets can be drawn from
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9. The LC droplets behave more or less like
at the lower salt concentration,
(see
Figure 8 and
Figure 9I). The LC comprises organic molecules with (i) a rigid core of two phenyl groups and (ii) a side chain of a cyano group (-CN
), making it polar. The cyano group in the LCs interacts with the dissociating positively charged ions of the PBS (Na
, K
, etc.) and the positively charged residues of the lysozyme. This interaction possibly impacts the packing of both lysozyme particles and LC droplets as more water evaporates during the drying process. Interestingly, we can see that the crack domains are larger than that of
, which is true for all the protein drops in the presence and absence of LCs. This indicates that the interactions between the salts–salts, salts–proteins, and proteins–proteins are rigid, and that they want to be together. It might also be the case that the presence of the salts has increased the film height, so the mechanical stress (via crack formation) acts differently from the drops without salts. This observation did not change when we added LCs into the system. This means LCs might not increase the film height but only affect the packing of the particles in the system. In other words, the LC droplets are somehow trapped in the layer between lysozyme particles and salts in the central regions. The evaporation of a further volume of water at the later drying stage (
0.7–0.8) propagates the bursting and random distribution of these droplets (see
Figure 12(BIV,BVI)). Unlike
, there is an additional salt layer on top of the LC distribution. In contrast, the peripheral region of the lysozyme+PBS+LC drop mostly contains the lysozyme particles, which drive the LCs to fill these cracked domains. Since the LCs do not have enough volume to fulfill the cracked domains, and the water leaves the system by that time, the LCs stay where they are. The whole process thus provides a plausible explanation of the reduced birefringence intensity (I under crossed polarizing configuration) when the
was increased despite having a fixed volume of LCs (see
Figure 9I–IV).