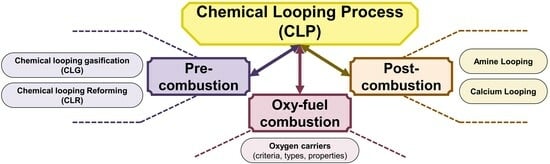
Chemical Looping Strategy in Various Types of Carbon Capture Technologies
Abstract
:1. Introduction
1.1. Carbon Capture Technologies: Types and Trend
1.2. Chemical Looping Process in Improvement of Carbon Capture Strategy
2. Chemical Looping Process in Pre-Combustion Strategies
2.1. Chemical Looping Gasification (CLG)
2.2. Chemical Looping Reforming (CLR)
3. Chemical Looping Process in Oxy-Fuel Combustion Strategies
3.1. Oxygen Carrier Materials
- High capacity in oxygen transport;
- Good properties for thermodynamic and kinetic capacity for conversion of fuel;
- Great endurance in melting temperature;
- Good characteristics in fluidization and low chance of agglomeration;
- High mechanical strength;
- Low possibility of carbon deposition;
- Low-cost and eco-friendly materials.
3.2. Metal-Oxide-Based Oxygen Carriers
3.2.1. Copper (Cu)
3.2.2. Nickel (Ni)
3.2.3. Cobalt (Co)
3.2.4. Manganese (Mn)
3.2.5. Iron (Fe)
3.3. Combined Metal Oxygen Carriers
4. Chemical Looping Process for Post-Combustion Strategies
4.1. Amine Looping
4.2. Ca-Looping
5. Application of Chemical Looping Process: Challenge and Alternative Solutions
5.1. Challenges
5.2. Alternative Solutions
6. Chemical Looping Process: Future and Prospect in Industrial Application
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peres, C.B.; Resende, P.M.R.; Nunes, L.J.R.; Morais, L.C.d. Advances in Carbon Capture and Use (CCU) Technologies: A Comprehensive Review and CO2 Mitigation Potential Analysis. Clean Technol. 2022, 4, 1193–1207. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand-Jahromi, S.; Sedghkerdar, M.H.; Mahinpey, N. A review of chemical looping combustion technology: Fundamentals, and development of natural, industrial waste, and synthetic oxygen carriers. Fuel 2023, 341, 127626. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Large scale application of carbon capture to process industries—A review. J. Clean. Prod. 2022, 362, 132300. [Google Scholar] [CrossRef]
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Fan, Y.; Liu, Q.; Wang, X.; Xu, K.; Jin, J.; Ma, J.; Ma, J. Mercury transformation and removal in chemical looping combustion of coal: A review. Fuel 2023, 347, 128440. [Google Scholar] [CrossRef]
- Li, F.; Zeng, L.; Fan, L.-S. Biomass direct chemical looping process: Process simulation. Fuel 2010, 89, 3773–3784. [Google Scholar] [CrossRef]
- Chai, S.Y.W.; Ngu, L.H.; How, B.S. Review of carbon capture absorbents for CO2 utilization. Greenh. Gases Sci. Technol. 2022, 12, 394–427. [Google Scholar] [CrossRef]
- Vorokhta, M.; Kusdhany, M.I.M.; Vöröš, D.; Nishihara, M.; Sasaki, K.; Lyth, S.M. Microporous carbon foams: The effect of nitrogen-doping on CO2 capture and separation via pressure swing adsorption. Chem. Eng. J. 2023, 471, 144524. [Google Scholar] [CrossRef]
- Bai, F.; Liu, X.; Liu, Y.; Li, M.; Sani, S.; Guo, W.; Sun, C. CO2 capture from dilute sources using triamine functionalized MCF silica at ambient temperature. Microporous Mesoporous Mater. 2023, 349, 112370. [Google Scholar] [CrossRef]
- Sanchez Quiñones, C.A.A.; Águeda Maté, V.I.; Delgado Dobladez, J.A.; Álvarez-Torrellas, S.; Larriba, M.; Martín-Martínez, M. Imines supported on silica as CO2 capture selective adsorbents. Chem. Eng. Res. Des. 2023, 194, 573–581. [Google Scholar] [CrossRef]
- González-Zamora, E.; Ibarra, I.A. CO2 capture under humid conditions in metal–organic frameworks. Mater. Chem. Front. 2017, 1, 1471–1484. [Google Scholar] [CrossRef]
- Ashkanani, H.E.; Wang, R.; Shi, W.; Siefert, N.S.; Thompson, R.L.; Smith, K.; Steckel, J.A.; Gamwo, I.K.; Hopkinson, D.; Resnik, K.; et al. Levelized Cost of CO2 Captured Using Five Physical Solvents in Pre-combustion Applications. Int. J. Greenh. Gas Control 2020, 101, 103135. [Google Scholar] [CrossRef]
- Siefert, N.S.; Agarwal, S.; Shi, F.; Shi, W.; Roth, E.A.; Hopkinson, D.; Kusuma, V.A.; Thompson, R.L.; Luebke, D.R.; Nulwala, H.B. Hydrophobic physical solvents for pre-combustion CO2 capture: Experiments, computational simulations, and techno-economic analysis. Int. J. Greenh. Gas Control 2016, 49, 364–371. [Google Scholar] [CrossRef]
- Hornbostel, K.; Nguyen, D.; Bourcier, W.; Knipe, J.; Worthington, M.; McCoy, S.; Stolaroff, J. Packed and fluidized bed absorber modeling for carbon capture with micro-encapsulated sodium carbonate solution. Appl. Energy 2019, 235, 1192–1204. [Google Scholar] [CrossRef]
- Zarei, A.; Hafizi, A.; Rahimpour, M.R.; Raeissi, S. Carbon dioxide absorption into aqueous potassium salt solutions of glutamine amino acid. J. Mol. Liq. 2020, 301, 111743. [Google Scholar] [CrossRef]
- Hasan, S.; Abbas, A.J.; Nasr, G.G. Improving the Carbon Capture Efficiency for Gas Power Plants through Amine-Based Absorbents. Sustainability 2021, 13, 72. [Google Scholar] [CrossRef]
- Lee, Y.; Moon, S.; Lee, S.; Seo, D.; Park, Y. Significance of wettability of porous media and its implication for hydrate-based pre-combustion CO2 capture. Chem. Eng. J. 2022, 446, 136832. [Google Scholar] [CrossRef]
- Arora, A.; Kumar, A.; Bhattacharjee, G.; Balomajumder, C.; Kumar, P. Hydrate-Based Carbon Capture Process: Assessment of Various Packed Bed Systems for Boosted Kinetics of Hydrate Formation. J. Energy Resour. Technol. 2020, 143, 033005. [Google Scholar] [CrossRef]
- Beckwée, E.J.; Watson, G.; Houlleberghs, M.; Arenas Esteban, D.; Bals, S.; Van Der Voort, P.; Breynaert, E.; Martens, J.; Baron, G.V.; Denayer, J.F.M. Enabling hydrate-based methane storage under mild operating conditions by periodic mesoporous organosilica nanotubes. Heliyon 2023, 9, e17662. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Lipiński, W. Research progress and challenges in hydrate-based carbon dioxide capture applications. Appl. Energy 2020, 269, 114928. [Google Scholar] [CrossRef]
- Owebor, K.; Diemuodeke, E.O.; Briggs, T.A. Thermo-economic and environmental analysis of integrated power plant with carbon capture and storage technology. Energy 2022, 240, 122748. [Google Scholar] [CrossRef]
- Font-Palma, C.; Cann, D.; Udemu, C. Review of Cryogenic Carbon Capture Innovations and Their Potential Applications. C J. Carbon Res. 2021, 7, 58. [Google Scholar] [CrossRef]
- Hou, R.; Wang, S.; Wang, L.; Li, C.; Wang, H.; Xu, Y.; Wang, C.; Pan, Y.; Xing, W. Enhanced CO2 separation performance by incorporating KAUST-8 nanosheets into crosslinked poly(ethylene oxide) membrane. Sep. Purif. Technol. 2023, 309, 123057. [Google Scholar] [CrossRef]
- Rezaee, Z.; Mohammadi, T.; Bakhtiari, O. Preparation of organic-filled compatible nanocomposite membranes for enhanced CO2 permselectivity. J. Ind. Eng. Chem. 2023, 126, 145–159. [Google Scholar] [CrossRef]
- Anggarini, U.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. An ultrahigh permeance and CO2 selective membrane of organosilica-based coordination polymer tailored via nickel crosslinking. J. Membr. Sci. 2023, 679, 121698. [Google Scholar] [CrossRef]
- Nie, M.; Zhou, C.; Feng, W.; Xin, C.; Yu, X.; Li, Q. Hierarchical ZnS layers-coated Ti3+-TiO2 nanostructures for boosted visible-light photocatalytic norfloxacin degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130814. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, J.; Huo, G.; Zhang, Z.; Zhou, X.; Bi, J.; Kang, S.; Dai, Z.; Li, N. Cross-linked PI membranes with simultaneously improved CO2 permeability and plasticization resistance via tunning polymer precursor orientation degree. J. Membr. Sci. 2023, 687, 121994. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Y.; Ma, H.; He, G.; Jiang, Z. Advanced organic molecular sieve membranes for carbon capture: Current status, challenges and prospects. Adv. Membr. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Ding, M.; Jiang, H.-L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal–Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Luo, L.; Ye, M.; Liu, W.; Zhu, T. Interactive adsorption mechanism and product distribution of impurity gases on CO2 adsorption over amine-grafted ZSM-5/SBA-16 adsorbent. Fuel 2023, 354, 129307. [Google Scholar] [CrossRef]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Paramio, C.; Palomar, J. Aspen plus supported design of pre-combustion CO2 capture processes based on ionic liquids. Sep. Purif. Technol. 2022, 290, 120841. [Google Scholar] [CrossRef]
- Li, C.; Zhao, T.; Yang, A.; Liu, F. Highly Efficient Absorption of CO2 by Protic Ionic Liquids-Amine Blends at High Temperatures. ACS Omega 2021, 6, 34027–34034. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Qi, C.; Zhao, H.; Wang, L.; Li, Q.; Wang, L. Loosely bonded dual-functionalized ionic liquid-based phase change solvent for energy-saving CO2 capture. Fuel 2023, 350, 128748. [Google Scholar] [CrossRef]
- Goel, A.; Moghaddam, E.M.; Liu, W.; He, C.; Konttinen, J. Biomass chemical looping gasification for high-quality syngas: A critical review and technological outlooks. Energy Convers. Manag. 2022, 268, 116020. [Google Scholar] [CrossRef]
- Liu, G.; Mao, X.; Yang, B.; Shang, J.; Wu, Z. Research progress on chemical looping reforming of macromolecular components of volatiles from biomass pyrolysis based on decoupling strategy. Fuel Process. Technol. 2022, 235, 107375. [Google Scholar] [CrossRef]
- Arnaiz del Pozo, C.; Cloete, S.; Cloete, J.H.; Jiménez Álvaro, Á.; Amini, S. The potential of chemical looping combustion using the gas switching concept to eliminate the energy penalty of CO2 capture. Int. J. Greenh. Gas Control 2019, 83, 265–281. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Li, L.; Sun, L. Zero-energy penalty carbon capture and utilization for liquid fuel and power cogeneration with chemical looping combustion. J. Clean. Prod. 2019, 235, 34–43. [Google Scholar] [CrossRef]
- Gayán, P.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Assessment of technological solutions for improving chemical looping combustion of solid fuels with CO2 capture. Chem. Eng. J. 2013, 233, 56–69. [Google Scholar] [CrossRef]
- Chiu, P.-C.; Ku, Y. Chemical Looping Process—A Novel Technology for Inherent CO2 Capture. Aerosol Air Qual. Res. 2012, 12, 1421–1432. [Google Scholar] [CrossRef]
- Lyngfelt, A. Chemical Looping Combustion: Status and Development Challenges. Energy Fuels 2020, 34, 9077–9093. [Google Scholar] [CrossRef]
- Cabello, A.; Mendiara, T.; Abad, A.; Adánez, J. Techno-economic analysis of a chemical looping combustion process for biogas generated from livestock farming and agro-industrial waste. Energy Convers. Manag. 2022, 267, 115865. [Google Scholar] [CrossRef]
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Avoiding CO2 capture effort and cost for negative CO2 emissions using industrial waste in chemical-looping combustion/gasification of biomass. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 1–24. [Google Scholar] [CrossRef]
- Adánez, J.; Gayán, P.; Adánez-Rubio, I.; Cuadrat, A.; Mendiara, T.; Abad, A.; García-Labiano, F.; de Diego, L.F. Use of Chemical-Looping processes for coal combustion with CO2 capture. Energy Procedia 2013, 37, 540–549. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Wang, Y.; Huo, R.; Huang, Z.; Liu, M.; Wei, G.; Zhao, Z.; Li, H.; Fang, Y. Review of Biomass Chemical Looping Gasification in China. Energy Fuels 2020, 34, 7847–7862. [Google Scholar] [CrossRef]
- Osman, M.; Khan, M.N.; Zaabout, A.; Cloete, S.; Amini, S. Review of pressurized chemical looping processes for power generation and chemical production with integrated CO2 capture. Fuel Process. Technol. 2021, 214, 106684. [Google Scholar] [CrossRef]
- Mohamed, U.; Zhao, Y.-j.; Yi, Q.; Shi, L.-j.; Wei, G.-q.; Nimmo, W. Evaluation of life cycle energy, economy and CO2 emissions for biomass chemical looping gasification to power generation. Renew. Energy 2021, 176, 366–387. [Google Scholar] [CrossRef]
- Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Chemical Looping Combustion: A Brief Overview. Energies 2022, 15, 1563. [Google Scholar] [CrossRef]
- Antzara, A.; Heracleous, E.; Bukur, D.B.; Lemonidou, A.A. Thermodynamic analysis of hydrogen production via chemical looping steam methane reforming coupled with in situ CO2 capture. Int. J. Greenh. Gas Control 2015, 32, 115–128. [Google Scholar] [CrossRef]
- Najera, M.; Solunke, R.; Gardner, T.; Veser, G. Carbon capture and utilization via chemical looping dry reforming. Chem. Eng. Res. Des. 2011, 89, 1533–1543. [Google Scholar] [CrossRef]
- Zhou, C.; Shah, K.; Song, H.; Zanganeh, J.; Doroodchi, E.; Moghtaderi, B. Integration Options and Economic Analysis of an Integrated Chemical Looping Air Separation Process for Oxy-fuel Combustion. Energy Fuels 2016, 30, 1741–1755. [Google Scholar] [CrossRef]
- Tang, Y.; You, F. Life cycle environmental and economic analysis of pulverized coal oxy-fuel combustion combining with calcium looping process or chemical looping air separation. J. Clean. Prod. 2018, 181, 271–292. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Gao, Z.; Fu, J.; Ao, W.; Dai, J. CO2 Capture with Chemical Looping Combustion of Gaseous Fuels: An Overview. Energy Fuels 2017, 31, 3475–3524. [Google Scholar] [CrossRef]
- High, M.; Patzschke, C.F.; Zheng, L.; Zeng, D.; Xiao, R.; Fennell, P.S.; Song, Q. Hydrotalcite-Derived Copper-Based Oxygen Carrier Materials for Efficient Chemical-Looping Combustion of Solid Fuels with CO2 Capture. Energy Fuels 2022, 36, 11062–11076. [Google Scholar] [CrossRef]
- Daneshmand-Jahromi, S.; Hashem Sedghkerdar, M.; Mahinpey, N. Synthesis, characterization, and kinetic study of nanostructured copper-based oxygen carrier supported on silica and zirconia aerogels in the cyclic chemical looping combustion process. Chem. Eng. J. 2022, 448, 137756. [Google Scholar] [CrossRef]
- Wang, K.; Liu, D.; Liu, L.; Liu, J.; Hu, X.; Li, P.; Li, M.; Vasenko, A.S.; Xiao, C.; Ding, S. Tuning the local electronic structure of oxygen vacancies over copper-doped zinc oxide for efficient CO2 electroreduction. eScience 2022, 2, 518–528. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Bu, H.; Xu, Z.; Zhao, H. Semi-continuous Operation of Chemical Looping Combustion of Coal Using a Low-Cost Composite Oxygen Carrier. Energy Fuels 2022, 36, 9450–9459. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Fernández, J.R.; Amieiro, A.; Rastogi, M.; Brandt, J.; Spallina, V. Lab-scale experimental demonstration of CaCu chemical looping for hydrogen production and in-situ CO2 capture from a steel-mill. Fuel Process. Technol. 2022, 237, 107475. [Google Scholar] [CrossRef]
- Cabello, A.; Abad, A.; Mendiara, T.; Izquierdo, M.T.; de Diego, L.F. Outstanding performance of a Cu-based oxygen carrier impregnated on alumina in chemical looping combustion. Chem. Eng. J. 2023, 455, 140484. [Google Scholar] [CrossRef]
- Liu, S.; He, F.; Zhao, K.; Zhao, H.; Huang, Z.; Wei, G.; Yang, W. Long-term coal chemical looping gasification using a bimetallic oxygen carrier of natural hematite and copper ore. Fuel 2022, 309, 122106. [Google Scholar] [CrossRef]
- Zeng, P.; Dou, B.; Zhang, H.; Wu, K.; Zhao, L.; Luo, C.; Chen, H.; Xu, Y. Chemical looping steam reforming of ethanol without and with in-situ CO2 capture. Int. J. Hydrogen Energy 2022, 47, 6552–6568. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, L.; Zhao, W.; Li, F.; Chen, X. Ni-Fe bimetallic hexaaluminate for efficient reduction of O2-containing CO2 via chemical looping. Chem. Eng. J. 2022, 441, 136071. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, Q.; Wei, G.; Zhang, Y.; Hou, Y.; Huang, Z.; Zheng, A.; Zhao, Z.; Li, H. Reaction performance and lattice oxygen migration of MnFe2O4 oxygen carrier in methane-carbon dioxide reaction system. Int. J. Hydrogen Energy 2020, 45, 30254–30266. [Google Scholar] [CrossRef]
- Hu, J.; Hongmanorom, P.; Galvita, V.V.; Li, Z.; Kawi, S. Bifunctional Ni-Ca based material for integrated CO2 capture and conversion via calcium-looping dry reforming. Appl. Catal. B Environ. 2021, 284, 119734. [Google Scholar] [CrossRef]
- Pachler, R.F.; Penthor, S.; Mayer, K.; Hofbauer, H. Investigation of the fate of nitrogen in chemical looping combustion of gaseous fuels using two different oxygen carriers. Energy 2020, 195, 116926. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Jin, B.; Liang, Z. Layered double hydroxide derived bifunctional Ca-Fe-Mg material for integrated CO2 capture and utilization via chemical looping strategy. Chem. Eng. J. 2022, 431, 133826. [Google Scholar] [CrossRef]
- Staničić, I.; Brorsson, J.; Hellman, A.; Mattisson, T.; Backman, R. Thermodynamic Analysis on the Fate of Ash Elements in Chemical Looping Combustion of Solid Fuels–Iron-Based Oxygen Carriers. Energy Fuels 2022, 36, 9648–9659. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Fang, S.; Jiang, H.; Huang, Z.; Wei, G.; Wang, X.; Zhao, Z.; Huang, H. Chemical looping combustion of lignite using iron ore modified by foreign ions: Alkaline-earth and transition metal ions. Fuel 2022, 327, 125079. [Google Scholar] [CrossRef]
- Iftikhar, S.; Martin, W.; Gao, Y.; Yu, X.; Wang, I.; Wu, Z.; Li, F. LaNixFe1−xO3 as flexible oxygen or carbon carriers for tunable syngas production and CO2 utilization. Catal. Today 2023, 416, 113854. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Z.; Cui, C.; Zhang, J.; Wei, J. Effect of Nickel and Cobalt Doping on the Redox Performance of SrFeO3-δ toward Chemical Looping Dry Reforming of Methane. Energy Fuels 2023, 37, 12045–12057. [Google Scholar] [CrossRef]
- Wang, B.; Li, H.; Wang, W.; Luo, C.; Mei, D. Chemical looping combustion of lignite with the CaSO4–CoO mixed oxygen carrier. J. Energy Inst. 2020, 93, 1229–1241. [Google Scholar] [CrossRef]
- Adánez-Rubio, I.; Nilsson, A.; Izquierdo, M.T.; Mendiara, T.; Abad, A.; Adánez, J. Cu-Mn oxygen carrier with improved mechanical resistance: Analyzing performance under CLC and CLOU environments. Fuel Process. Technol. 2021, 217, 106819. [Google Scholar] [CrossRef]
- Ksepko, E.; Lysowski, R. Effective direct chemical looping coal combustion using bimetallic Ti-supported Fe2O3-MnO2 oxygen carriers. Greenh. Gases Sci. Technol. 2023, 13, 575–592. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-based oxygen carriers in chemical looping conversions: A review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Narindri Rara Winayu, B.; Li, J.-D.; Chu, H. Fe-based oxygen carrier for the chemical looping combustion of CO, H2, and CH4 syngas in fluidized bed reactor under interruption of H2S. Chem. Eng. Res. Des. 2023, 194, 514–528. [Google Scholar] [CrossRef]
- Narindri Rara Winayu, B.; Li, C.-T.; Chu, H. Effective performance of ilmenite oxygen carrier for chemical looping combustion of carbon monoxide, hydrogen, and methane in a fluidized bed reactor. J. Clean. Prod. 2022, 379, 134881. [Google Scholar] [CrossRef]
- Khakpoor, N.; Mostafavi, E.; Mahinpey, N.; De la Hoz Siegler, H. Oxygen transport capacity and kinetic study of ilmenite ores for methane chemical-looping combustion. Energy 2019, 169, 329–337. [Google Scholar] [CrossRef]
- Keller, M.; Oka, H.; Otomo, J. Reactivity improvement of ilmenite by calcium nitrate melt infiltration for Chemical Looping Combustion of biomass. Carbon Resour. Convers. 2019, 2, 51–58. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Izquierdo, M.T.; Adánez, J. Chemical Looping Combustion of gaseous and solid fuels with manganese-iron mixed oxide as oxygen carrier. Energy Convers. Manag. 2018, 159, 221–231. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; Izquierdo, M.T.; Gayán, P.; de Diego, L.F.; Adánez, J. Evaluation of Mn-Fe mixed oxide doped with TiO2 for the combustion with CO2 capture by Chemical Looping assisted by Oxygen Uncoupling. Appl. Energy 2019, 237, 822–835. [Google Scholar] [CrossRef]
- Abad, A.; Pérez-Vega, R.; de Diego, L.F.; Gayán, P.; Izquierdo, M.T.; García-Labiano, F.; Adánez, J. Thermochemical assessment of chemical looping assisted by oxygen uncoupling with a MnFe-based oxygen carrier. Appl. Energy 2019, 251, 113340. [Google Scholar] [CrossRef]
- Narindri Rara Winayu, B.; Li, B.-H.; Chu, H. Fe2O3/TiO2 oxygen carrier for chemical looping combustion of CO, H2, and CH4 in a fluidized bed reactor. Mater. Today Commun. 2022, 32, 104026. [Google Scholar] [CrossRef]
- Zhao, F.; Cui, C.; Dong, S.; Xu, X.; Liu, H. An overview on the corrosion mechanisms and inhibition techniques for amine-based post-combustion carbon capture process. Sep. Purif. Technol. 2023, 304, 122091. [Google Scholar] [CrossRef]
- Chirone, R.; Paulillo, A.; Coppola, A.; Scala, F. Carbon capture and utilization via calcium looping, sorption enhanced methanation and green hydrogen: A techno-economic analysis and life cycle assessment study. Fuel 2022, 328, 125255. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Sedghkerdar, M.H.; Mahinpey, N. Calcium looping carbon capture: Progress and prospects. Can. J. Chem. Eng. 2022, 100, 2140–2171. [Google Scholar] [CrossRef]
- Chen, S.; Qin, C.; Yin, J.; Zhou, X.; Chen, S.; Ran, J. Understanding sulfation effect on the kinetics of carbonation reaction in calcium looping for CO2 capture. Fuel Process. Technol. 2021, 221, 106913. [Google Scholar] [CrossRef]
- Carbone, C.; Ferrario, D.; Lanzini, A.; Stendardo, S.; Agostini, A. Evaluating the Carbon Footprint of Cement Plants Integrated with the Calcium Looping CO2 Capture Process. Front. Sustain. 2022, 3, 809231. [Google Scholar] [CrossRef]
- Ferrario, D.; Stendardo, S.; Verda, V.; Lanzini, A. Solar-driven calcium looping system for carbon capture in cement plants: Process modelling and energy analysis. J. Clean. Prod. 2023, 394, 136367. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.; Wang, H.; Du, Y.; Ma, J.; Zhang, X.; Wang, Z. Carbon Capture and Storage: History and the Road Ahead. Engineering 2022, 14, 33–43. [Google Scholar] [CrossRef]
- Khan, M.N.; Chiesa, P.; Cloete, S.; Amini, S. Integration of chemical looping combustion for cost-effective CO2 capture from state-of-the-art natural gas combined cycles. Energy Convers. Manag. X 2020, 7, 100044. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Fan, M.; Wang, Y.; Adidharma, H.; Gasem, K.A.M.; Radosz, M. A cost-effective approach to reducing carbon deposition and resulting deactivation of oxygen carriers for improvement of energy efficiency and CO2 capture during methane chemical-looping combustion. Appl. Energy 2017, 193, 381–392. [Google Scholar] [CrossRef]
- Olabi, A.G.; Obaideen, K.; Elsaid, K.; Wilberforce, T.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Assessment of the pre-combustion carbon capture contribution into sustainable development goals SDGs using novel indicators. Renew. Sustain. Energy Rev. 2022, 153, 111710. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in carbon capture technologies: A review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Hong, W.Y. A techno-economic review on carbon capture, utilisation and storage systems for achieving a net-zero CO2 emissions future. Carbon Capture Sci. Technol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Gabrielli, P.; Campos, J.; Becattini, V.; Mazzotti, M.; Sansavini, G. Optimization and assessment of carbon capture, transport and storage supply chains for industrial sectors: The cost of resilience. Int. J. Greenh. Gas Control 2022, 121, 103797. [Google Scholar] [CrossRef]
- Bartocci, P.; Abad, A.; Mattisson, T.; Cabello, A.; Loscertales, M.d.l.O.; Negredo, T.M.; Zampilli, M.; Taiana, A.; Serra, A.; Arauzo, I.; et al. Bioenergy with Carbon Capture and Storage (BECCS) developed by coupling a Pressurised Chemical Looping combustor with a turbo expander: How to optimize plant efficiency. Renew. Sustain. Energy Rev. 2022, 169, 112851. [Google Scholar] [CrossRef]
- Soosaiprakasam, I.R.; Veawab, A. Corrosion inhibition performance of copper carbonate in MEA-CO2 capture unit. Energy Procedia 2009, 1, 225–229. [Google Scholar] [CrossRef]
- Emori, W.; Jiang, S.L.; Duan, D.L.; Ekerenam, O.O.; Zheng, Y.G.; Okafor, P.C.; Qiao, Y.X. Corrosion behavior of carbon steel in amine-based CO2 capture system: Effect of sodium sulfate and sodium sulfite contaminants. Mater. Corros. 2017, 68, 674–682. [Google Scholar] [CrossRef]
- Narindri Rara Winayu, B.; Shen, N.-Y.; Chu, H. Valuable reutilization of Basic Oxygen Furnace (BOF) slag for the CO2 sorption from oxy-fuel combustion in a fluidized bed reactor. J. CO2 Util. 2022, 66, 102280. [Google Scholar] [CrossRef]
- Shirmohammadi, R.; Aslani, A.; Ghasempour, R. Challenges of carbon capture technologies deployment in developing countries. Sustain. Energy Technol. Assess. 2020, 42, 100837. [Google Scholar] [CrossRef]
- Tahir, F.; Saeed, M.A.; Ali, U. Biomass energy perspective in Pakistan based on chemical looping gasification for hydrogen production and power generation. Int. J. Hydrogen Energy 2023, 48, 18211–18232. [Google Scholar] [CrossRef]
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical looping beyond combustion—A perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- Khallaghi, N.; Abbas, S.Z.; Manzolini, G.; De Coninck, E.; Spallina, V. Techno-economic assessment of blast furnace gas pre-combustion decarbonisation integrated with the power generation. Energy Convers. Manag. 2022, 255, 115252. [Google Scholar] [CrossRef]
- Wang, J.; Wu, P.; Wei, Y.; Zhao, Q.; Ning, P.; Huang, Y.; Wen, S.; Xu, J.; Wang, Q. Study of calcium-based CO2 sorbent with high cycling stability derived from steel slag and its anti-sintering mechanism. J. CO2 Util. 2022, 66, 102279. [Google Scholar] [CrossRef]
- Zhou, C.; Shah, K.; Moghtaderi, B. Techno-Economic Assessment of Integrated Chemical Looping Air Separation for Oxy-Fuel Combustion: An Australian Case Study. Energy Fuels 2015, 29, 2074–2088. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Li, L.; Rao, D. Life-cycle assessment of SNG and power generation: The role of implement of chemical looping combustion for carbon capture. Energy 2019, 172, 777–786. [Google Scholar] [CrossRef]
- Bortuzzo, V.; Bertagna, S.; Bucci, V. Mitigation of CO2 Emissions from Commercial Ships: Evaluation of the Technology Readiness Level of Carbon Capture Systems. Energies 2023, 16, 3646. [Google Scholar] [CrossRef]
- Mi, R.; Pan, G. Slowing down CO2 effective diffusion speeds in recycled aggregate concrete by using carbon capture technology and high-quality recycled aggregate. J. Build. Eng. 2022, 45, 103628. [Google Scholar] [CrossRef]
- Chisalita, D.-A.; Cormos, C.-C. Techno-economic assessment of hydrogen production processes based on various natural gas chemical looping systems with carbon capture. Energy 2019, 181, 331–344. [Google Scholar] [CrossRef]
- Cormos, C.-C. Hydrogen production from fossil fuels with carbon capture and storage based on chemical looping systems. Int. J. Hydrogen Energy 2011, 36, 5960–5971. [Google Scholar] [CrossRef]
- Hu, Q.; Shen, Y.; Chew, J.W.; Ge, T.; Wang, C.-H. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem. Eng. J. 2020, 379, 122346. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Kar, S.; Goeppert, A.; Prakash, G.K.S. Integrated CO2 Capture and Conversion to Formate and Methanol: Connecting Two Threads. Acc. Chem. Res. 2019, 52, 2892–2903. [Google Scholar] [CrossRef]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019, 31, 1804257. [Google Scholar] [CrossRef]
- Liu, M.; Yi, Y.; Wang, L.; Guo, H.; Bogaerts, A. Hydrogenation of Carbon Dioxide to Value-Added Chemicals by Heterogeneous Catalysis and Plasma Catalysis. Catalysts 2019, 9, 275. [Google Scholar] [CrossRef]


| No | Technologies | Working Principle | Consideration of Implementation | References |
|---|---|---|---|---|
| 1 | Adsorption | Applied several types of solid material (zeolites, clay, oxides, etc.) as selective adsorbent for CO2. | Can be applied under low energy demand but highly influenced by the affinity of CO2, along with the molar size, molar weight, and polarity of the sorbents. | [9,10,11,12] |
| 2 | Physical absorption | Implements high pressure and low temperature to improve solubility of CO2 into the sorption medium. | Unsuitable for low CO2 content. Further test on performance and stability are required. | [13,14] |
| 3 | Chemical absorption | Utilizes several types of chemicals (amines, potassium carbonate, calcium carbonate, amino acids, etc.) to absorb the CO2 gas stream. | Can be applied in low pressure condition, but regeneration of solvent needs to be maintained. Created the issue of corrosion and high regeneration energy demand. | [15,16,17] |
| 4 | Hydrate-based | Deliver the CO2 containing flue gas using high pressure into water to form hydrate. | Applicable for pre- and post-combustion but highly depends on the rate of hydrate formation, solubility, and contact surface area. | [18,19,20,21] |
| 5 | Cryogenic separation | Use the difference in sublimation properties and high boiling points for physical separation of CO2 in gas. | High energy demand for conditioning of refrigeration cycle. High applicability for CO2 concentration > 50%. | [22,23] |
| 6 | Membrane separation | Supply the CO2 gas stream trough membrane materials to | Membrane materials with high permeability, low cost, and high durability are required. Considered as an eco-friendly and low energy demand strategy but low partial pressure issue should be handled. | [24,25,26,27] |
| 7 | Molecular sieve | Gas separation strategy using permeable materials with suitable hole size of the CO2 gas molecule. | Simple process for adsorption or desorption, but the design is complicated, and the process is highly influenced by the thermal stability. | [28,29,30] |
| 8 | Metal organic frameworks | Capture CO2 by the open structure of porous solid of metal organic materials. | Maintenance of practical pressure, CO2 partial pressure, and temperature is essential to reach high efficiency. Adaptive to be combined with other capture methods. | [31,32] |
| 9 | Ionic liquids | CO2 sorption using the combination of anion and cation made of organic or inorganic liquids. | Non-corrosivity and low volatility are accompanied by high viscosity and cost during the process. High possibility to be combined with the membrane strategy. | [33,34,35] |
| No | Oxygen Carrier | Features | References |
|---|---|---|---|
| 1 | Cu-based mixed oxides from hydrotalcite derivation (CuAl2O4, CuAlO2, etc.) | Improved oxygen uptake and release capacity. | [55] |
| 2 | Cu-based with silica and zirconia support | Improved stability during the cycle test. | [56] |
| 3 | Cu-doped Zn oxides (Cu-ZnO) | Improved the CO Faradaic efficiency and current density for the process of CO2 electrochemical reduction. | [57] |
| 4 | Cu-based with red mud support (Cu13.0Red87.0@C) | Improved capacity of carbon capture using low-cost material. | [58] |
| 5 | Ca-Cu-based oxygen carrier | Improved capacity and stability in simultaneous H2 production and CO2 capture from chemical looping system. | [59] |
| 6 | Cu-Al-based oxygen carrier | Promoted mechanical strength during chemical looping combustion process | [60] |
| 7 | Hematite- and copper-ore-based oxygen carrier | Improved selectivity, stability, and ability of oxygen release. | [61] |
| 8 | 20NiO/MgAl2O4 oxygen carrier | Improved the hydrogen selectivity and material stability in chemical looping steam reforming system. | [62] |
| 9 | LaFe2.9Ni0.1Al9O19 | Promoted the utilization of CO2 and separation of O2 impurities in chemical looping system | [63] |
| 10 | MnFe2O4 oxygen carrier | Formation of spinel structure improved the migration of lattice oxygen | [64] |
| 11 | Ni-Ca-based co-loaded on ZrO2 | Improved isothermal capture and release of CO2 | [65] |
| 12 | Perovskite (CaMn0.775Mg0.1Ti0.125O3-δ)- and Cu-based oxygen carrier | Promoted reduction of NOx emission during CO2 capture | [66] |
| 13 | Ca-Fe-Mg oxygen carrier | Created a stable H2/CO ratio due to the presence of Ca | [67] |
| 14 | Fe-based oxygen carrier (K0.85Fe0.85Ti0.15O2, K0.4Fe0.4Ti0.6O2, KTi8O16, and KTi8O16.5) | Improved thermostability during CO2 capture in CLC system | [68] |
| 15 | Iron ore combined with alkaline earth and transition metal ions | Improved the reactivity with volatiles for production of higher CO2 content | [69] |
| 16 | LaFe1−xNixO3-δ oxygen carrier | Formed simple tuning for syngas production and CO2 utilization | [70] |
| 17 | Ni and Co doping on SrFeO3-δ oxygen carrier | Increased the migration of lattice oxygen for chemical looping dry reforming of methane | [71] |
| 18 | Combination of lignite with CaSO4-CoO oxygen carrier | Improved reactivity and reduce sintering effect in chemical looping combustion | [72] |
| 19 | Cu-Mn oxygen carrier | Prolonged lifetime and increase mechanical resistance during chemical looping combustion system | [73] |
| 20 | Fe2O3-MnO2 on Ti support | Promoted physicochemical stability during the looping cycle | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narindri Rara Winayu, B.; Tseng, T.-K.; Chu, H. Chemical Looping Strategy in Various Types of Carbon Capture Technologies. Processes 2023, 11, 3164. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11113164
Narindri Rara Winayu B, Tseng T-K, Chu H. Chemical Looping Strategy in Various Types of Carbon Capture Technologies. Processes. 2023; 11(11):3164. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11113164
Chicago/Turabian StyleNarindri Rara Winayu, Birgitta, Ting-Ke Tseng, and Hsin Chu. 2023. "Chemical Looping Strategy in Various Types of Carbon Capture Technologies" Processes 11, no. 11: 3164. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11113164