Biosynthesis of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from Different 4-Hydroxybutyrate Precursors by New Wild-Type Strain Cupriavidus necator IBP/SFU-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Culture Medium and Cultivation Conditions
2.3. PHA Recovery from Cell Biomass
2.4. PHA Chemical Composition
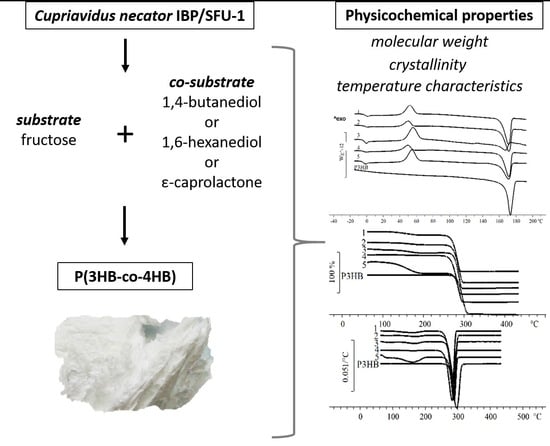
2.5. Physicochemical Properties of PHAs
2.6. Statistics
3. Results
3.1. Effect of ε-Caprolactone as a 4HB Precursor on the Biomass Concentration, Content and Composition of the Polymer Obtained in the Culture of the New Strain Cupriavidus necator IBP/SFU-1
3.2. Effect of 1,4-Butanediol as a Precursor of 4HB on the Biomass Concentration, Content and Composition of the Polymer Obtained in the Culture of the New Strain Cupriavidus necator IBP/SFU-1
3.3. Effect of 1,6-Hexanediol as a 4HB Precursor on the Biomass Concentration, Content and Composition of the Polymer Obtained in the Culture of the New Strain Cupriavidus necator IBP/SFU-1
3.4. Physicochemical Properties of P(3HB-co-4HB) Copolymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciesielski, S.; Możejko, J.; Przybyłek, G. The influence of nitrogen limitation on mcl-PHA synthesis by two newly isolated strains of Pseudomonas sp. J. Ind. Microbiol. Biotechnol. 2010, 37, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Sagong, H.Y.; Son, H.F.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Structural insights into polyhydroxyalkanoates biosynthesis. Trends Biochem. Sci. 2018, 43, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Slaninova, E.; Fritz, I.; Daffert, C.; Meixner, K.; Sedrlova, Z.; Koller, M. Novel unexpected functions of PHA granules. Appl. Microbiol. Biotechnol. 2020, 104, 4795–4810. [Google Scholar] [CrossRef]
- Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for biofuel and biorefineries. Polymers 2021, 13, 253. [Google Scholar] [CrossRef]
- Heo, K.; Yoon, J.; Jin, K.S.; Jin, S.; Sato, H.; Ozaki, Y.; Satkowski, M.M.; Noda, I.; Ree, M. Structural evolution in microbial polyesters. J. Phys. Chem. B 2008, 112, 4571–4582. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, A.; Owen, A. Dielectric relaxation spectroscopy of poly [(R)-3-hydroxybutyrate](PHB) during crystallization. Polym. Int. 2004, 53, 863–868. [Google Scholar] [CrossRef]
- Noda, I.; Green, P.R.; Satkowski, M.M.; Schechtman, L.A. Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 2005, 6, 580–586. [Google Scholar] [CrossRef]
- Luo, R.; Xu, K.; Chen, G.Q. Study of miscibility, crystallization, mechanical properties, and thermal stability of blends of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-4-hydroxybutyrate). J. Appl. Polym. Sci. 2007, 105, 3402–3408. [Google Scholar] [CrossRef]
- Koller, M.; Hesse, P.; Bona, R.; Kutschera, C.; Atlić, A.; Braunegg, G. Biosynthesis of high quality polyhydroxyalkanoate co-and terpolyesters for potential medical application by the archaeon Haloferax mediterranei. Macromol. Symp. 2007, 253, 33–39. [Google Scholar] [CrossRef]
- Shrivastav, A.; Kim, H.Y.; Kim, Y.R. Advances in the applications of polyhydroxyalkanoate nanoparticles for novel drug delivery system. BioMed Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef]
- Vigneswari, S.; Amirul, A.A. Biodegradability and cellular compatibility of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) via subcutaneous implantation in rat model. Malay. Appl. Biol. 2017, 46, 205–212. [Google Scholar]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Doi, Y.; Segawa, A.; Kunioka, M. Biosynthesis and characterization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int. J. Biol. Macromol. 1990, 12, 106–111. [Google Scholar] [CrossRef]
- Lee, W.H.; Azizan, M.N.M.; Sudesh, K. Effects of culture conditions on the composition of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) synthesized by Comamonas acidovorans. Polym. Degrad. Stab. 2004, 84, 129–134. [Google Scholar] [CrossRef]
- Chanprateep, S.; Buasri, K.; Muangwong, A.; Utiswannakul, P. Biosynthesis and biocompatibility of biodegradable poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 2003–2012. [Google Scholar] [CrossRef]
- Hsieh, W.; Mitomo, H.; Kasuya, K.I.; Komoto, T. Enzymatic degradation and aminolysis of microbial poly (3-hydroxybutyrate-co-4-hydroxybutyrate) single crystals. J. Polym. Environ. 2006, 14, 79–87. [Google Scholar] [CrossRef]
- Saito, Y.; Doi, Y. Microbial synthesis and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int. J. Biol. Macromol. 1994, 16, 99–104. [Google Scholar] [CrossRef]
- Saito, Y.; Nakamura, S.; Hiramitsu, M.; Doi, Y. Microbial synthesis and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Int. 1996, 39, 169–174. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Doi, Y.; Kanesawa, Y.; Kunioka, M.; Saito, T. Biodegradation of microbial copolyesters: Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1990, 23, 26–31. [Google Scholar] [CrossRef]
- Doi, Y.; Kunioka, M.; Nakamura, Y.; Soga, K. Nuclear magnetic resonance studies on unusual bacterial copolyesters of 3-hydroxybutyrate and 4-hydroxybutyrate. Macromolecules 1988, 21, 2722–2727. [Google Scholar] [CrossRef]
- Kang, C.K.; Kusaka, S.; Doi, Y. Structure and properties of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Alcaligenes latus. Biotechnol. Lett. 1995, 17, 583–588. [Google Scholar] [CrossRef]
- Renner, G.; Haage, G.; Braunegg, G. Production of short-side-chain polyhydroxyalkanoates by various bacteria from the rRNA superfamily III. Appl. Microbiol. Biotechnol. 1996, 46, 268–272. [Google Scholar] [CrossRef]
- Choi, M.H.; Yoon, S.C.; Lenz, R.W. Production of poly (3-hydroxybutyric acid-co-4-hydroxybutyric acid) and poly (4-hydroxybutyric acid) without subsequent degradation by Hydrogenophaga pseudoflava. Appl. Environ. Microbiol. 1999, 65, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, T.T.; Gomez, J.G.C.; Buffoni, E.; Sánchez Rodriguez, R.J.; Schripsema, J.; Lopes, M.S.G.; Silva, L.F.D. Exploring the potential of Burkholderia sacchari to produce polyhydroxyalkanoates. J. Appl. Microbiol. 2014, 116, 815–829. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.; Van Keulen, F.; Ferreira, B.S.; Telo, J.P.; da Fonseca, M.M.R. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) by Burkholderia sacchari using wheat straw hydrolysates and gamma-butyrolactone. Int. J. Biol. Macromol. 2014, 71, 59–67. [Google Scholar] [CrossRef]
- Pospisilova, A.; Vodicka, J.; Trudicova, M.; Juglova, Z.; Smilek, J.; Mencik, P.; Masilko, J.; Slaninova, E.; Melcova, V.; Kalina, M.; et al. Effects of differing monomer compositions on properties of P(3HB-co-4HB) synthesized by Aneurinibacillus sp. H1 for various applications. Polymers 2022, 14, 2007. [Google Scholar] [CrossRef]
- Valentin, H.E.; Dennis, D. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in recombinant Escherichia coli grown on glucose. J. Biotechnol. 1997, 58, 33–38. [Google Scholar] [CrossRef]
- Li, Z.J.; Shi, Z.Y.; Jian, J.; Guo, Y.Y.; Wu, Q.; Chen, G.Q. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metabol. Eng. 2010, 12, 352–359. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Jiang, X.; Chen, G.Q. Engineering Escherichia coli for enhanced production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) in larger cellular space. Metabol. Eng. 2014, 25, 183–193. [Google Scholar] [CrossRef]
- Lv, L.; Ren, Y.L.; Chen, J.C.; Wu, Q.; Chen, G.Q. Application of CRISPRi for prokaryotic metabolic engineering involving multiple genes, a case study: Controllable P (3HB-co-4HB) biosynthesis. Metabol. Eng. 2015, 29, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Bryant, D.A. Metabolic engineering of Synechococcus sp. PCC 7002 to produce poly-3-hydroxybutyrate and poly-3-hydroxybutyrate-co-4-hydroxybutyrate. Metabol. Eng. 2015, 32, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, J.; Ye, J.; Zhang, H.; Che, X.; Ma, Y.; Li, M.; Wu, L.P.; Chen, G.Q. Engineering Halomonas bluephagenesis TD01 for non-sterile production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Biores. Technol. 2017, 244, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, A.; Zaleha, Z.; Yahya, A.R.; Majid, M.I.; Amirul, A.A. Production of copolymer poly (3-hydroxybutyrate-co-4-hydroxybutyrate) through a one-step cultivation process. World J. Microbiol. Biotechnol. 2008, 24, 2403–2409. [Google Scholar] [CrossRef]
- Rao, U.; Sridhar, R.; Sehgal, P.K. Biosynthesis and biocompatibility of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Cupriavidus necator from spent palm oil. Biochem. Eng. J. 2010, 49, 13–20. [Google Scholar] [CrossRef]
- Ramachandran, H.; Amirul, A.A. Yellow-pigmented Cupriavidus sp., a novel bacterium capable of utilizing glycerine pitch for the sustainable production of P (3HB-co-4HB). J. Chem. Technol. Biotechnol. 2012, 88, 1030–1038. [Google Scholar] [CrossRef]
- Huong, K.H.; Mohd Yahya, A.R.; Amirul, A.A. Pronounced synergistic influence of mixed substrate cultivation on single step copolymer P (3HB-co-4HB) biosynthesis with a wide range of 4HB monomer composition. J. Chem. Technol. Biotechnol. 2013, 89, 1023–1029. [Google Scholar] [CrossRef]
- Chai, H.; Ahmad, R.; Yahya, A.; Majid, M.; Amirul, A. Microbial synthesis of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer by Cupriavidus sp. USMAA2-4 through a two step cultivation process. Afr. J. Biotechnol. 2009, 8, 4189–4196. [Google Scholar]
- Vigneswari, S.; Vijaya, S.; Majid, M.I.A.; Sudesh, K.; Sipaut, C.S.; Azizan, M.N.M.; Amirul, A.A. Enhanced production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer with manipulated variables and its properties. J. Ind. Microbiol. Biotechnol. 2009, 36, 547–556. [Google Scholar] [CrossRef]
- Chai, J.U.N.M.; Krishnan, S.H.R.; Hang, V.T.Y.; Hamdan, H.A.M.; Ruzelan, N.N.; Vigneswari, S. Effects of various carbon precursors combination in regulating the molar fraction of P (3HB-co-4HB) using locally isolated Cupriavidus sp. TMT11. Malays. Appl. Biol. 2020, 49, 79–84. [Google Scholar] [CrossRef]
- Huong, K.H.; Kannusamy, S.; Lim, S.Y.H.; Amirul, A.A. Biosynthetic enhancement of single-stage poly (3-hydroxybutyrate-co-4-hydroxybutyrate) production by manipulating the substrate mixtures. J. Ind. Microbiol. Biotechnol. 2015, 42, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Huong, K.H.; The, C.H.; Amirul, A.A. Microbial-based synthesis of highly elastomeric biodegradable poly (3-hydroxybutyrate-co-4-hydroxybutyrate) thermoplastic. Int. J. Biol. Macromol. 2017, 101, 983–995. [Google Scholar] [CrossRef]
- de Macedo, M.A.; Oliveira-Filho, E.R.; Taciro, M.K.; Piccoli, R.A.M.; Gomez, J.G.C.; Silva, L.F. Poly (3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] biotechnological production: Challenges and opportunities. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; Raposo, R.S.; de Almeida, M.C.M.; Cesário, M.T.; Sevrin, C.; Grandfils, C.; Da Fonseca, M.M.R. Effect of cultivation parameters on the production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) and poly (3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Biores. Technol. 2012, 111, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.; Shishatskaya, E. Properties of PHA bi-, ter-, and quarter-polymers containing 4-hydroxybutyrate monomer units. Int. J. Biol. Macromol. 2018, 111, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Amirul, A.A.; Yahya, A.R.M.; Sudesh, K.; Azizan, M.N.M.; Majid, M.I.A. Biosynthesis of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer by Cupriavidus sp. USMAA1020 isolated from Lake Kulim, Malaysia. Biores. Technol. 2008, 99, 4903–4909. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, B.H.; Kim, B.S. Production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) by Ralstonia eutropha. Biochem. Eng. J. 2005, 23, 169–174. [Google Scholar] [CrossRef]
- Song, S.; Hein, S.; Steinbüchel, A. Production of poly (4-hydroxybutyric acid) by fed-batch cultures of recombinant strains of Escherichia coli. Biotechnol. Lett. 1999, 21, 193–197. [Google Scholar] [CrossRef]
- Valentin, H.E.; Zwingmann, G.; Schönebaum, A.; Steinbüchel, A. Metabolic pathway for biosynthesis of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from 4-hydroxybutyrate by Alcaligenes eutrophus. Eur. J. Biochem. 1995, 227, 43–60. [Google Scholar] [CrossRef]
- Volova, T.; Demidenko, A.; Kiselev, E.; Baranovskiy, S.; Shishatskaya, E.; Zhila, N. Polyhydroxyalkanoate synthesis based on glycerol and implementation of the process under conditions of pilot production. Appl. Microbiol. Biotechnol. 2019, 103, 225–237. [Google Scholar] [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Nemtsev, I.V.; Lukyanenko, A.V. Production and properties of microbial polyhydroxyalkanoates synthesized from hydrolysates of Jerusalem artichoke tubers and vegetative biomass. Polymers 2022, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Shishatskaya, E.I.; Volova, T.G. Sugar beet molasses as a potential C-substrate for PHA production by Cupriavidus necator. Bioengineering 2022, 9, 154. [Google Scholar] [CrossRef]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Vasiliev, A.D.; Nemtsev, I.V.; Shishatskaya, E.I.; Volova, T.G. Properties of degradable polyhydroxyalkanoates (PHAs) synthesized by a new strain, Cupriavidus necator IBP/SFU-1, from various carbon sources. Polymers 2021, 13, 3142. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A submersion method for culture of hydrogen-oxidizing bacteria: Growth physiological studies. Arch. Microbiol. 1961, 38, 209–222. [Google Scholar]
- Volova, T.; Kiselev, E.; Shishatskaya, E.; Zhila, N.; Boyandin, A.; Syrvacheva, D.; Vinogradova, O.; Kalacheva, G.; Vasiliev, A.; Peterson, I. Cell growth and PHA accumulation from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Ermakov, A.I.; Arasimovich, V.V.; Smirnova-Ikonnikova, M.I.; Yarosh, N.P.; Lukovnikova, G.A. Metody Biokhimicheskogo Issledovaniya Rastenii (Methods of Biochemical Plant Research); Kolos: Leningrad, Russia, 1972; 456p. (In Russian) [Google Scholar]
- Kiselev, E.G. Technical and Technological Bases of Biosynthesis of Reserve Polyhydroxyalkanoates by Hydrogen Bacteria. Ph.D. Thesis, Siberian Federal University, Krasnoyarsk, Russia, 2012. [Google Scholar]
- Braunegg, G.; Sonnleitner, B.Y.; Lafferty, R.M. A rapid gas chromatographic method for the determination of poly-β-hydroxybutyric acid in microbial biomass. Europ. J. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable PHAs with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef]
- Mitomo, H.; Hsieh, W.C.; Nishiwaki, K.; Kasuya, K.; Doi, Y. Poly (3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Comamonas acidovorans. Polymer 2001, 42, 3455–3461. [Google Scholar] [CrossRef]
- Iqbal, N.; Amirul, A.A. Synthesis of P (3HB-co-4HB) copolymer with target-specific 4HB molar fractions using combinations of carbon substrates. J. Chem. Technol. Biotechnol. 2013, 89, 407–418. [Google Scholar] [CrossRef]
- Gomez, J.G.C.; Rodrigues, M.F.A.; Alli, R.C.P.; Torres, B.B.; Bueno Netto, C.L.; Silva, L.F. Evaluation of soil gram-negative bacteria yielding polyhydroxyalkanoic acids from carbohydrates and propionic acid. Appl. Microbiol. Biotechnol. 1996, 45, 785–791. [Google Scholar] [CrossRef]
- Miranda De Sousa Dias, M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-batch synthesis of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from sucrose and 4-hydroxybutyrate precursors by Burkholderia sacchari strain DSM 17165. Bioengineering 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Wada, Y.; Chang, C.P. Fermentation, biodegradation and tensile strength of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) synthesized by Delftia acidovorans. J. Taiwan Inst. Chem. Eng. 2009, 40, 143–147. [Google Scholar] [CrossRef]
- Chanprateep, S.; Kulpreecha, S. Production and characterization of biodegradable terpolymer poly (3-hydroxybutyrate-co-3-hydroxyvalerate-co-4-hydroxybutyrate) by Alcaligenes sp. A-04. J. Biosci. Bioeng. 2006, 101, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, P.; Pernicova, I.; Novackova, I.; Kourilova, X.; Kalina, M.; Kovalcik, A.; Koller, M.; Nebesarova, J.; Krzyzanek, V.; Hrubanova, K.; et al. Introducing the newly isolated bacterium Aneurinibacillus sp. H1 as an auspicious thermophilic producer of various polyhydroxyalkanoates (PHA) copolymers–2. Material study on the produced copolymers. Polymers 2020, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Norhafini, H.; Huong, K.H.; Amirul, A.A. High PHA density fed-batch cultivation strategies for 4HB-rich P (3HB-co-4HB) copolymer production by transformant Cupriavidus malaysiensis USMAA1020. Int. J. Biol. Macromol. 2019, 125, 1024–1032. [Google Scholar] [CrossRef]
- Syafiq, I.M.; Huong, K.H.; Shantini, K.; Vigneswari, S.; Abd Aziz, N.; Amirul, A.A.A.; Bhubalan, K. Synthesis of high 4-hydroxybutyrate copolymer by Cupriavidus sp. transformants using one-stage cultivation and mixed precursor substrates strategy. Enzyme Microb. Technol. 2017, 98, 1–8. [Google Scholar] [CrossRef]
- Kunioka, M.; Tamaki, A.; Doi, Y. Crystalline and thermal properties of bacterial copolyesters: Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1989, 22, 694–697. [Google Scholar] [CrossRef]
- Ye, J.; Huang, W.; Wang, D.; Chen, F.; Yin, J.; Li, T.; Zhang, H.; Chen, G.Q. Pilot scale-up of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) production by Halomonas bluephagenesis via cell growth adapted optimization process. Biotechnol. J. 2018, 13, 1800074. [Google Scholar] [CrossRef]
- Ramachandran, H.; Amirul, A.A. Bioconversion of glycerine pitch into a novel yellow-pigmented P(3HB-co-4HB) copolymer: Synergistic effect of ammonium acetate and polymer characteristics. Appl. Biochem. Biotechnol. 2014, 172, 891–909. [Google Scholar] [CrossRef]
- Jo, M.; Jang, Y.; Lee, E.; Shin, S.; Kang, H.-J. The modification of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by melt blending. Polymers 2022, 14, 1725. [Google Scholar] [CrossRef]





| Co-Substrate | Polymer Yields (YP/S, g PHA/g Substrate) | % of Maximum Theoretical YP/S |
|---|---|---|
| ε-caprolactone | 0.13–0.15 | 27–31% |
| 1,4-butanediol | 0.09–0.14 | 19–29% |
| 1,6-hexanediol | 0.11–0.13 | 23–27% |
| N | Composition of Monomers, mol.% | Mn, kDa | Mw, kDa | Ð | Cx, % | Tmelt, °C | Tdegr, °C | Tg, °C | Tc °C | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3HB | 4HB | |||||||||
| 1 | 88.7 | 11.3 | 47 | 292 | 6.21 | 68 | 171.1 | 124.3 271.3 | –3.3 | 56.2 51.8 |
| 2 | 86.0 | 14.0 | 67 | 352 | 5.25 | 67 | 171.1 | 137.2 274.6 | –3.4 | 60.4 49.8 |
| 3 | 84.0 | 16.0 | 58 | 295 | 5.09 | 64 | 127.5 167.1 | 154.1 276.7 | –4.4 | 55.7 |
| 4 | 81.4 | 18.6 | 49 | 329 | 6.71 | 62 | 171.1 | 269.3 | –4.8 | 65.0 49.5 |
| 5 | 79.4 | 22.4 | 51 | 272 | 5.33 | 59 | 170.9 | 134.6 271.4 | –5.7 | 54.3 |
| 6 | 100 | 0 | 158 | 436 | 2.76 | 72 | 173 | 292 | 4.7 | 84.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Biosynthesis of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from Different 4-Hydroxybutyrate Precursors by New Wild-Type Strain Cupriavidus necator IBP/SFU-1. Processes 2023, 11, 1423. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11051423
Zhila NO, Sapozhnikova KY, Kiselev EG, Shishatskaya EI, Volova TG. Biosynthesis of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from Different 4-Hydroxybutyrate Precursors by New Wild-Type Strain Cupriavidus necator IBP/SFU-1. Processes. 2023; 11(5):1423. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11051423
Chicago/Turabian StyleZhila, Natalia O., Kristina Yu. Sapozhnikova, Evgeniy G. Kiselev, Ekaterina I. Shishatskaya, and Tatiana G. Volova. 2023. "Biosynthesis of Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from Different 4-Hydroxybutyrate Precursors by New Wild-Type Strain Cupriavidus necator IBP/SFU-1" Processes 11, no. 5: 1423. https://0-doi-org.brum.beds.ac.uk/10.3390/pr11051423