Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of pH
Abstract
:1. Introduction
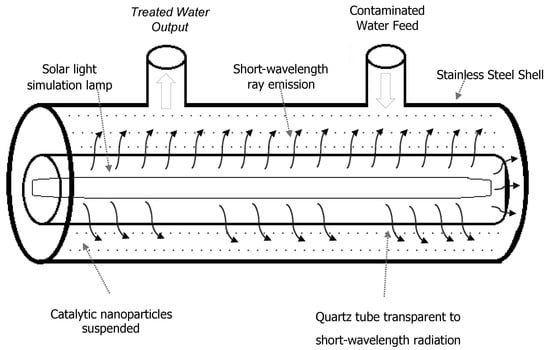
2. Materials and Methods
3. Results and Discussion
3.1. Photodegradation of Paracetamol
3.2. Paracetamol Photodegradation at pH 6.5
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970. [Google Scholar] [CrossRef] [Green Version]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Uheida, A.; Mohamed, A.; Belaqziz, M.; Nasser, W.S. Photocatalytic degradation of Ibuprofen, Naproxen, and Cetirizine using PAN-MWCNT nanofibers crosslinked TiO2-NH2 nanoparticles under visible light irradiation. Sep. Purif. Technol. 2019, 212, 110–118. [Google Scholar] [CrossRef]
- Tobajas, M.; Belver, C.; Rodriguez, J.J. Degradation of emerging pollutants in water under solar irradiation using novel TiO2-ZnO/clay nanoarchitectures. Chem. Eng. J. 2017, 309, 596–606. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Sun, P.; Liu, J.; Ji, Y.; Nakada, N.; Qiao, Z.; Tanaka, H.; Yang, Y. Nanomaterials for treating emerging contaminants in water by adsorption and photocatalysis: Systematic review and bibliometric analysis. Sci. Total Environ. 2018, 627, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Richardson, S.D.; Kimura, S.Y. Emerging environmental contaminants: Challenges facing our next generation and potential engineering solutions. Environ. Technol. Innov. 2017, 8, 40–56. [Google Scholar] [CrossRef]
- Sanganyado, E.; Gwenzi, W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.F.; Ingerslev, F.; Lützhøft, H.C.H.; Jørgensen, S.E. Occurrence, fate and effects of pharmaceutical substances in the environment-A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Barber, L.B.; Keefe, S.H.; Brown, G.K.; Furlong, E.T.; Gray, J.L.; Kolpin, D.W.; Meyer, M.T.; Sandstrom, M.W.; Zaugg, S.D. Persistence and potential effects of complex organic contaminant mixtures in wastewater-impacted streams. Environ. Sci. Technol. 2013, 47, 2177–2188. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Newson, R.B.; Ring, S.M.; Rose-Zerilli, M.J.; Holloway, J.W.; Henderson, A.J. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J. Allergy Clin. Immunol. 2010, 126. [Google Scholar] [CrossRef] [PubMed]
- Bedner, M.; MacCrehan, W.A. Transformation of acetaminophen by chlorination produces the toxicants 1,4-benzoquinone and N-acetyl-p-benzoquinone imine. Environ. Sci. Technol. 2006, 40, 516–522. [Google Scholar] [CrossRef]
- Xagoraraki, I.; Hullman, R.; Song, W.; Li, H.; Voice, T. Effect of pH on degradation of acetaminophen and production of 1,4-benzoquinone in water chlorination. J. Water Supply Res. Technol.-AQUA 2008, 57, 381–390. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Vogna, D. Paracetamol oxidation from aqueous solutions by means of ozonation and H2O2/UV system. Water Res. 2003, 37, 993–1004. [Google Scholar] [CrossRef]
- Valdez, H.C.A.; Jiménez, G.G.; Granados, S.G.; de León, C.P. Degradation of paracetamol by advance oxidation processes using modified reticulated vitreous carbon electrodes with TiO2 and CuO/TiO2/Al2O3. Chemosphere 2012, 89, 1195–1201. [Google Scholar] [CrossRef]
- Jagannathan, M.; Grieser, F.; Ashokkumar, M. Sonophotocatalytic degradation of paracetamol using TiO2 and Fe3+. Sep. Purif. Technol. 2013, 103, 114–118. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Fu, W.; Tsang, D.C.W.; Yang, X. The roles of halides in the acetaminophen degradation by UV/H2O2 treatment: Kinetics, mechanisms, and products analysis. Chem. Eng. J. 2015, 271, 214–222. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Zhou, S.; Xiao, Y.; Zhuang, Z. Kinetic study of acetaminophen degradation by UV-based advanced oxidation processes. Chem. Eng. J. 2014, 253, 229–236. [Google Scholar] [CrossRef]
- Su, C.C.; Cada, C.A.; Dalida, M.L.P.; Lu, M.C. Effect of UV light on acetaminophen degradation in the electro-Fenton process. Sep. Purif. Technol. 2013, 120, 43–51. [Google Scholar] [CrossRef]
- Martinez-Haya, R.; Miranda, M.A.; Marin, M.L. Type I vs Type II photodegradation of pollutants. Catal. Today 2018, 313, 161–166. [Google Scholar] [CrossRef]
- Scott, J.P.; Ollis, D.F. Integration of chemical and biological oxidation processes for water treatment: Review and recommendations. Environ. Prog. 1995, 14, 88–103. [Google Scholar] [CrossRef]
- Verlicchi, P.; al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Baird, C.; Cann, M. Enviromental Chemistry, 5th ed.; W. H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Wen, Z.H.; Chen, L.; Meng, X.Z.; Duan, Y.P.; Zhang, Z.S.; Zeng, E.Y. Occurrence and human health risk of wastewater-derived pharmaceuticals in a drinking water source for Shanghai, East China. Sci. Total Environ. 2014, 490, 987–993. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of Titanium Oxide Nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Idakiev, V.; Yuan, Z.-Y.; Tabakova, T.; Su, B.-L. Titanium oxide nanotubes as supports of nano-sized gold catalysts for low temperature water-gas shift reaction. Appl. Catal. A Gen. 2005, 281, 149–155. [Google Scholar] [CrossRef]
- Ou, H.H.; Lo, S.L. Review of titania nanotubes synthesized via the hydrothermal treatment: Fabrication, modification, and application. Sep. Purif. Technol. 2007, 58, 179–191. [Google Scholar] [CrossRef]
- Guo, Y.; Lee, N.H.; Oh, H.J.; Yoon, C.R.; Park, K.S.; Lee, W.H.; Li, Y.; Lee, H.G.; Lee, K.S.; Kim, S.J. Preparation of titanate nanotube thin film using hydrothermal method. Thin Solid Films 2008, 516, 8363–8371. [Google Scholar] [CrossRef]
- Li, H.L.; Luo, W.L.; Tian, W.Y.; Chen, T.; Li, C.; Sun, M.; Zhu, D.; Liu, R.R.; Zhao, Y.L.; Liu, C.L. Fabrication and Photocatalytic Activity of Pt-Inserted Titania Nanotubes. Spectrosc. Spectr. Anal. 2009, 29, 1623–1626. [Google Scholar] [CrossRef]
- Zavala, M.L.; Morales, S.L. Synthesis of stable TiO2 nanotubes: Effect of hydrothermal treatment, acid washing and annealing temperature. Heliyon 2017, 3, e00456. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.E.; Pernas, E.; Saiers, J. Influence of mineralization products on the coagulation of TiO2 photocatalyst. Langmuir 1999, 15, 2071–2076. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Photocatalytic oxidation of paracetamol: Dominant reactants, intermediates, and reaction mechanisms. Environ. Sci. Technol. 2009, 43, 460–465. [Google Scholar] [CrossRef]
- Zavala, M.Á.L.; Lara, C.R.J. Degradation of Paracetamol and Its Oxidation Products in Surface Water by Electrochemical Oxidation. Environ. Eng. Sci. 2018, 35, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Zavala, M.Á.L.; Estrada, E.E. Degradation of acetaminophen and its transformation products in aqueous solutions by using an electrochemical oxidation cell with stainless steel electrodes. Water 2016, 8, 383. [Google Scholar] [CrossRef]
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Fundamentals of Biological Treatment. In Wastewater Engineering Treatment Reuse; McGraw-Hill: New York, NY, USA, 2003; pp. 611–615, 619–621. [Google Scholar]
- Zhang, X.; Wu, F.; Wu, X.; Chen, P.; Deng, N. Photodegradation of acetaminophen in TiO2 suspended solution. J. Hazard. Mater. 2008, 157, 300–307. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir-Hinshelwood kinetics-A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Ollis, D.F. Kinetics of liquid phase photocatalyzed reactions: An illuminating approach. J. Phys. Chem. B 2005, 109, 2439–2444. [Google Scholar] [CrossRef]
- El-shahawy, A. DFT Cancer Energy Barrier and Spectral Studies of Aspirin, Paracetamol and Some Analogues. Comput. Chem. 2014, 2, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Shourong, Z.; Qingguo, H.; Jun, Z.; Bingkun, W. A study on dye photoremoval in TiO2 suspension solution. J. Photochem. Photobiol. A Chem. 1997, 108, 235–238. [Google Scholar] [CrossRef]
- Kulkarni, M.; Flašker, A.; Lokar, A.; Mrak-Poljšak, M.; Mazare, A.; Artenjak, A.; Čučnik, S.; Kralj, S.; Velikonja, A.; Schmuki, P.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar] [CrossRef]
- Borges, M.; García, D.; Hernández, T.; Ruiz-Morales, J.; Esparza, P. Supported Photocatalyst for Removal of Emerging Contaminants from Wastewater in a Continuous Packed-Bed Photoreactor Configuration. Catalysts 2015, 5, 77–87. [Google Scholar] [CrossRef] [Green Version]





| pH | K′ (min−1) | R2 | t1/2 (min) | Efficiency after 180 min (%) |
|---|---|---|---|---|
| 2.5 | 0.016 | 0.9571 | 88.6 | 46 |
| 4.5 | 0.023 | 0.9305 | 60.1 | 67 |
| 5.5 | 0.029 | 0.9039 | 47.8 | 83 |
| 6.5 | 0.034 | 0.9641 | 27.4 | 99 |
| 7.5 | 0.027 | 0.9375 | 51.3 | 71 |
| 8.5 | 0.024 | 0.8422 | 49.9 | 68 |
| 10.5 | 0.008 | 0.8958 | 136.8 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Morales, S.A.; Morales, G.; López Zavala, M.Á.; Arce-Sarria, A.; Machuca-Martínez, F. Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of pH. Processes 2019, 7, 319. https://0-doi-org.brum.beds.ac.uk/10.3390/pr7060319
Lozano-Morales SA, Morales G, López Zavala MÁ, Arce-Sarria A, Machuca-Martínez F. Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of pH. Processes. 2019; 7(6):319. https://0-doi-org.brum.beds.ac.uk/10.3390/pr7060319
Chicago/Turabian StyleLozano-Morales, S. Alejandro, Graciela Morales, Miguel Ángel López Zavala, Augusto Arce-Sarria, and Fiderman Machuca-Martínez. 2019. "Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of pH" Processes 7, no. 6: 319. https://0-doi-org.brum.beds.ac.uk/10.3390/pr7060319