Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Electrochemical Characterization
3. Results and Discussions
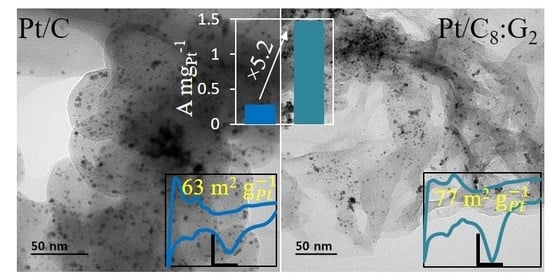
3.1. Surface Analysis of Pt/Cx:G10−x
3.2. Raman Analysis of Pt/Cx:G10−x
3.3. SSA Measurement of Pt/Cx:G10−x
3.4. XPS Analysis of Pt/Cx:G10−x
3.5. Electrochemical Analysis of Pt/Cx:G10−x
3.6. Electrochemical ORR on Pt/Cx:G10−x
3.7. Kinetics of ORR on Pt/Cx:G10−x
3.8. Stability and Selectivity Towards ORR on Pt/Cx:G10−x
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of Pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Lim, B.; Lee, E.P.; Xia, Y.N. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ahmed, M.S.; Jeon, S. Electrochemical deposition of silver on manganese dioxide coated reduced graphene oxide for enhanced oxygen reduction reaction. J. Power Sources 2015, 288, 261. [Google Scholar] [CrossRef]
- Markovic, N.M.; Grgur, B.N.; Ross, P.N. Temperature-dependent hydrogen electrochemistry on platinum low-index single-crystal surfaces in acid solutions. J. Phys. Chem. B 1997, 101, 5405–5413. [Google Scholar] [CrossRef]
- Paulus, U.A.; Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kim, D.; Jeon, S. Covalently grafted platinum nanoparticles to multi walled carbon nanotubes for enhanced electrocatalytic oxygen reduction. Electrochimca Acta 2013, 92, 168. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.E.; Ahmed, M.S.; Jeon, S. 3,4-ethylenedioxythiophene functionalized graphene with palladium nanoparticles for enhanced electrocatalytic oxygen reduction reaction. J. Power Sources 2015, 281, 211. [Google Scholar] [CrossRef]
- Dong, Y.; Zhou, Y.W.; Wang, M.Z.; Zheng, S.L.; Jiang, K.; Cai, W.B. Facile aqueous phase synthesis of carbon supported B-doped Pt3Ni nanocatalyst for efficient oxygen reduction reaction. Electrochimca Acta 2017, 246, 242. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. Highly active graphene-supported NixPd100−x binary alloyed catalysts for electro-oxidation of ethanol in an alkaline media. ACS Catal. 2014, 4, 1830. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Lee, D.W.; Kim, Y.B. Carbon nanotubes-based PdM bimetallic catalysts through N4-system for efficient ethanol oxidation and hydrogen evolution reaction. Sci. Rep. 2019, 9, 11051. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.J.; Liu, L.; Lin, X.X.; Yuan, J.; Feng, J.J. One-pot synthesis of 3D freestanding porous PtAg hollow chain-like networks as efficient electrocatalyst for oxygen reduction reaction. Electrochimca Acta 2017, 245, 883. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. A novel δ-MnO2 with carbon nanotubes nanocomposite as an enzyme-free sensor for hydrogen peroxide electrosensing. RSC Adv. 2016, 6, 50572. [Google Scholar] [CrossRef]
- Bregoli, L.J. The influence of platinum crystallite size on the electrochemical reduction of oxygen in phosphoric acid. Electrochimca Acta 1978, 23, 489–492. [Google Scholar] [CrossRef]
- Kinoshita, K. Particle size effects for oxygen reduction on highly dispersed platinum in acid electrolytes. J. Electrochem. Soc. 1990, 137, 845–848. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhao, N.; Fang, B.; Li, H.; Bi, X.T.; Wang, H. Carbon-supported Pt-based alloy electrocatalysts for the oxygen reduction reaction in polymer electrolyte membrane fuel cells: Particle size, shape, and composition manipulation and their impact to activity. Chem. Rev. 2015, 115, 3433–3467. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today 2009, 4, 143–164. [Google Scholar] [CrossRef]
- Darling, R.M.; Meyers, J.P. Kinetic model of platinum dissolution in PEMFCs. J. Electrochem. Soc. 2003, 150, A1523–A1527. [Google Scholar] [CrossRef]
- Van der Vliet, D.F.; Wang, C.; Li, D.; Paulikas, A.P.; Greeley, J.; Rankin, R.B.; Strmcnik, D.; Tripkovic, D.; Markovic, N.M.; Stamenkovic, V.R. Unique electrochemical adsorption properties of Pt-skin surfaces. Angew. Chem. Int. Ed. 2012, 51, 3139–3142. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behavior during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Markovic, N.M.; Stamenkovic, V.R. Advanced platinum alloy electrocatalysts for the oxygen reduction reaction. ACS Catal. 2012, 2, 891–898. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, S.; Chang, Q.; Su, D.; Yue, J.; Du, Z.; Shao, M. Palladium−platinum core−shell electrocatalysts for oxygen reduction reaction prepared with the assistance of citric acid. ACS Catal. 2016, 6, 3428–3432. [Google Scholar] [CrossRef]
- Yang, J.; Kim, S.H.; Kwak, S.K.; Song, H.K. Curvature-induced metal-support interaction of an islands-byislands composite of platinum catalyst and carbon nano-onion for durable oxygen reduction. ACS Appl. Mater. Interfaces 2017, 9, 23302–23308. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Ahmed, M.S.; Cho, S.; Jeon, S. Simultaneous reduction and nitrogen functionalization of graphene oxide using lemon for metal-free oxygen reduction reaction. J. Power Sources 2017, 372, 116–124. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Han, H.S.; Jeon, S. One-step chemical reduction of graphene oxide with oligothiophene for improved electrocatalytic oxygen reduction reactions. Carbon 2013, 61, 164–172. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. New Approach for porous chitosan−graphene matrix preparation through enhanced amidation for synergic detection of dopamine and uric acid. ACS Omega 2017, 2, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Kim, Y.B. Amide-functionalized graphene with 1,4-diaminobutane as efficient metal-free and porous electrocatalyst for oxygen reduction. Carbon 2017, 111, 577–586. [Google Scholar] [CrossRef]
- Hu, C.; Xue, J.; Dong, L.; Jiang, Y.; Wang, X.; Qu, L.; Dai, L. Scalable preparation of multifunctional fire retardant ultralight graphene foams. ACS Nano 2016, 10, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Men, B.; Sun, Y.; Li, M.; Hu, C.; Zhang, M.; Wang, L.; Tang, Y.; Chen, Y.; Wan, P.; Pan, J. Hierarchical Metal-free nitrogen-doped porous graphene/carbon composites as an efficient oxygen reduction reaction catalyst. ACS Appl. Mater. Interfaces 2016, 8, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Wang, C.; Zhang, H.; Lu, C.; Wang, J. Hierarchical porous graphene carbon-based supercapacitors. Chem. Mater. 2015, 27, 2107–2113. [Google Scholar] [CrossRef]
- Higgins, D.C.; Hoque, M.A.; Hassan, F.; Choi, J.Y.; Kim, B.; Chen, Z. Oxygen reduction on graphene−carbon nanotube composites doped sequentially with nitrogen and sulfur. ACS Catal. 2014, 4, 2734–2740. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Probst, N.; Grivei, E. Structure and electrical properties of carbon black. Carbon 2002, 40, 201–205. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.; Huo, J.; Wang, S.; Dai, L. Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Sanetuntikul, J.; Hang, T.; Shanmugam, S. Hollow nitrogen-doped carbon spheres as efficient and durable electrocatalysts for oxygen reduction. Chem. Commun. 2014, 50, 9473–9476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wei, Z.; Gou, X. Nitrogen and phosphorus dual-doped graphene/carbon nanosheets as bifunctional electrocatalysts for oxygen reduction and evolution. ACS Catal. 2015, 5, 4133–4142. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. Electrochemical activity evaluation of chemically damaged carbon nanotube with palladium nanoparticles for ethanol oxidation. J. Power Sources 2015, 282, 479–488. [Google Scholar] [CrossRef]
- You, J.M.; Ahmed, M.S.; Han, H.S.; Choe, J.E.; Üstündag, Z.; Jeon, S. New approach of nitrogen and sulfur-doped graphene synthesis using dipyrrolemethane and their electrocatalytic activity for oxygen reduction in alkaline media. J. Power Sources 2015, 275, 73–79. [Google Scholar] [CrossRef]
- Joo, Y.; Ahmed, M.S.; Han, H.S.; Jeon, S. Preparation of electrochemically reduced graphene oxide-based silver-cobalt alloy nanocatalysts for efficient oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 21751–21761. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Rivera, L.M.; Fajardo, S.; Arévalo, M.C.; García, G.; Pastor, E. S-and N-doped graphene nanomaterials for the oxygen reduction reaction. Catalysts 2017, 7, 278–290. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, X.; Mao, S.; Bo, Z.; Kim, H.; Cui, S.; Lu, G.; Feng, X.; Chen, J. Crumpled nitrogen-doped graphene nanosheets with ultrahigh pore volume for high-performance supercapacitor. Adv. Mater. 2012, 24, 5610–5616. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. Highly efficient dual active palladium nanonetwork electrocatalyst for ethanol oxidation and hydrogen evolution. ACS Appl. Mater. Interfaces 2017, 9, 39303–39311. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kim, Y.B. 3D graphene preparation via covalent amide functionalization for efficient metal-free electrocatalysis in oxygen reduction. Sci. Rep. 2017, 7, 43279. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Ahmed, M.S.; Cho, S.; Jeon, S. Freestanding palladium nanonetworks electrocatalyst for oxygen reduction reaction in fuel cells. Int. J. Hydrogen Energy 2018, 43, 229–238. [Google Scholar] [CrossRef]
- Han, H.S.; Ahmed, M.S.; Jeong, H.; Jeon, S. Electrochemical sensing of monohydric alcohols on different linkers imbedded in between graphene and platinum nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Park, D.; Jeon, S. Ultrasmall PdmMn1_mOx binary alloyed nanoparticles on graphene catalysts for ethanol oxidation in alkaline media. J. Power Sources 2016, 308, 180–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhao, Y.; Wang, F.; Song, Y. Pt–Co secondary solid solution nanocrystals supported on carbon as next-generation catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 20086–20091. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Lee, D.W.; Kim, Y.B. Graphene supported silver nanocrystals preparation for efficient oxygen reduction in alkaline fuel cells. J. Electrochem. Soc. 2016, 163, F1169–F1176. [Google Scholar] [CrossRef]
- Wang, C.H.; Huang, H.C.; Chang, S.T.; Lin, Y.C.; Huang, M.F. Pyrolysis of melamine-treated vitamin B12 as a non-precious metal catalyst for oxygen reduction reaction. RSC Adv. 2014, 4, 4207–4211. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. New functionalized graphene sheets for enhanced oxygen reduction as metal-free cathode electrocatalysts. J. Power Sources 2012, 218, 168–173. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeong, H.; You, J.M.; Jeon, S. Electrocatalytic reduction of dioxygen at a modified glassy carbon electrode based on Nafion-dispersed single-walled carbon nanotubes and cobalt–porphyrin with palladium nanoparticles in acidic media. Electrochimca Acta 2011, 56, 4924–4929. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Jeon, S. The nanostructure of nitrogen atom linked carbon nanotubes with platinum employed to the electrocatalytic oxygen reduction. J. Nanosci. Nanotechnol. 2013, 13, 306. [Google Scholar] [CrossRef]
- Yun, M.; Ahmed, M.S.; Jeon, S. Thiolated graphene oxide-supported palladium cobalt alloyed nanoparticles as high performance electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 293, 380–387. [Google Scholar] [CrossRef]
- Tang, X.; Ng, H.Y. Cobalt and nitrogen-doped carbon catalysts for enhanced oxygen reduction and power production in microbial fuel cells. Electrochimca Acta 2017, 247, 193. [Google Scholar] [CrossRef]
- Sohn, G.J.; Choi, H.J.; Jeon, I.Y.; Chang, D.W.; Dai, L.; Baek, J.B. Water-dispersible, sulfonated hyperbranched poly(ether-ketone) grafted multiwalled carbon nanotubes as oxygen reduction catalysts. ACS Nano 2012, 6, 6345–6355. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ahmed, M.S.; Jeon, S. Covalent functionalization of graphene with 1,5-diaminonaphthalene and ultrasmall palladium nanoparticles for electrocatalytic oxygen reduction. Int. J. Hydrogen Energy 2017, 42, 2061–2070. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sui, X.L.; Huang, G.S.; Gu, D.M.; Wang, Z.B. Hierarchical carbon coated molybdenum dioxide nanotubes as a highly active and durable electrocatalytic support for methanol oxidation. J. Mater. Chem. A 2017, 5, 4067–4074. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, H.; Kim, Y.-B. Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells. Processes 2019, 7, 586. https://0-doi-org.brum.beds.ac.uk/10.3390/pr7090586
Begum H, Kim Y-B. Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells. Processes. 2019; 7(9):586. https://0-doi-org.brum.beds.ac.uk/10.3390/pr7090586
Chicago/Turabian StyleBegum, Halima, and Young-Bae Kim. 2019. "Improvement of Catalytic Activity of Platinum Nanoparticles Decorated Carbon Graphene Composite on Oxygen Electroreduction for Fuel Cells" Processes 7, no. 9: 586. https://0-doi-org.brum.beds.ac.uk/10.3390/pr7090586