Water Vapor Pathways during Freeze-Drying of Foamed Product Matrices Stabilized by Maltodextrin at Different Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurement of Dielectric Constant
2.4. Foam Preparation
2.5. Determination of Bubble Size Distribution and Overrun
2.6. Examination of Freeze-Drying Behavior Using a Freeze-Drying Microscope
2.7. Microwave-Supported Freeze-Drying
2.8. Determination of the Residual Water Content
2.9. Determination of Water Activity
2.10. Determination of the Diffusion Coefficient
2.11. Statistical Analysis
3. Results and Discussion
3.1. Microwave-Supported Freeze-Drying
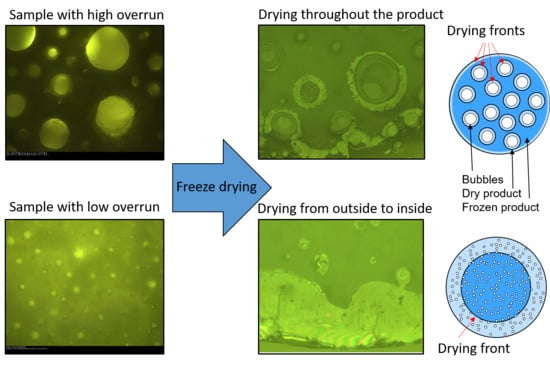
3.2. Freeze-Drying Microscope Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pikal, M.J. Freeze-drying of proteins. Part I: Process design. BioPharm 1990, 3, 18–27. [Google Scholar]
- Pikal, M.J. Freeze-drying of proteins. Part II: Formulation selection. BioPharm 1990, 3, 26–30. [Google Scholar]
- Flickinger, M.C. Downstream Industrial Biotechnology. In Recovery and Purification; Wiley: Hoboken, NJ, USA, 2013; ISBN 9781118131244. [Google Scholar]
- Schmitt, S. The Freeze Drying Challenge. Pharm. Technol. Eur. 2012, 24, 18-18. [Google Scholar]
- Ambros, S.; Dombrowski, J.; Boettger, D.; Kulozik, U. The Concept of Microwave Foam Drying under Vacuum: A Gentle Preservation Method for Sensitive Biological Material. J. Food Sci. 2019, 84, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, Z.-H.; Zeng, G.-M.; Wang, H.-L.; Yan, J.-W.; Xu, H.-Y.; Gou, C.-L. A novel approach for improving the drying behavior of sludge by the appropriate foaming pretreatment. Water Res. 2015, 68, 667–669. [Google Scholar] [CrossRef]
- Ozcelik, M.; Ambros, S.; Heigl, A.; Dachmann, E.; Kulozik, U. Impact of hydrocolloid addition and microwave processing condition on drying behavior of foamed raspberry puree. J. Food Eng. 2019, 240, 83–91. [Google Scholar] [CrossRef]
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Soponronnarit, S. Effects of foaming agents and foam density on drying characteristics and textural property of banana foams. LWT Food Sci. Technol. 2012, 47, 348–357. [Google Scholar] [CrossRef]
- Hajare, A.; More, H.; Pisal, S. Vacuum foam drying: New technology for preservation of sensitive biomolecules. Pharmatimes 2006, 38, 28–30. [Google Scholar]
- Ratti, C.; Kudra, T. Foam-mat drying: Energy and cost analyses. Can. Biosyst. Eng. 2006, 48, 27–32. [Google Scholar]
- Kadam, D.M.; Balasubramanian, S. Foam mat drying of tomato juice. J. Food Process. Preserv. 2011, 35, 488–495. [Google Scholar] [CrossRef]
- Ratti, C.; Kudra, T. Drying of Foamed Biological Materials: Opportunities and Challenges. Dry. Technol. 2007, 24, 1101–1108. [Google Scholar] [CrossRef]
- Ozcelik, M.; Heigl, A.; Kulozik, U.; Ambros, S. Effect of hydrocolloid addition and microwave-assisted freeze drying on the characteristics of foamed raspberry puree. Innov. Food Sci. Emerg. Technol. 2019, 56, 102183. [Google Scholar] [CrossRef]
- Lobo, F.A.; Nascimento, M.A.; Domingues, J.R.; Falcão, D.Q.; Hernanz, D.; Heredia, F.J.; de Lima Araujo, K.G. Foam mat drying of Tommy Atkins mango: Effects of air temperature and concentrations of soy lecithin and carboxymethylcellulose on phenolic composition, mangiferin, and antioxidant capacity. Food Chem. 2017, 221, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, P.; Kailappan, R.; Viswanathan, R.; Raghavan, G. Drying characteristics of foamed alphonso mango pulp in a continuous type foam mat dryer. J. Food Eng. 2007, 79, 1452–1459. [Google Scholar] [CrossRef]
- Walters, R.H.; Bhatnagar, B.; Tchessalov, S.; Izutsu, K.-I.; Tsumoto, K.; Ohtake, S. Next generation drying technologies for pharmaceutical applications. J. Pharm. Sci. 2014, 103, 2673–2695. [Google Scholar] [CrossRef]
- Sochanski, J.S.; Goyette, J.; Bose, T.K.; Akyel, C.; Bosisio, R. Freeze dehydration of foamed milk by microwaves. Dry. Technol. 1990, 8, 1017–1037. [Google Scholar] [CrossRef]
- Wang, W.; Hu, D.; Pan, Y.; Zhao, Y.; Chen, G. Freeze-drying of aqueous solution frozen with prebuilt pores. AIChE J. 2015, 61, 2048–2057. [Google Scholar] [CrossRef]
- Davies, J.D. Freeze-Dried Foam Dosage Form. U.S. Patent 4,642,903, 17 February 1987. [Google Scholar]
- Wertheim, J.H.; Mishkin, A.R. Freeze Drying a Frozen Foam of Coffee Concentrate. U.S. Patent 4,565,706, 21 January 1986. [Google Scholar]
- Raharitisfa, N.; Ratti, C. Foam-mat freeze-drying of apple juice Part 2: Stability of dry producs during storage. J. Food Process Eng. 2010, 33, 341–364. [Google Scholar] [CrossRef]
- Muthukumaran, A.; Ratti, C.; Raghavan, V.G.S. Foam-Mat Freeze Drying of Egg White—Mathematical Modeling Part II: Freeze Drying and Modeling. Dry. Technol. 2008, 26, 513–518. [Google Scholar] [CrossRef]
- Liapis, A.I.; Bruttini, R. A mathematical model for the spray freeze drying process: The drying of frozen particles in trays and in vials on trays. Int. J. Heat Mass Transfer 2009, 52, 100–111. [Google Scholar] [CrossRef]
- Chitu, T.; Vessot, S.; Peczalski, R.; Andrieu, J.; Woinet, B.; Françon, A. Influence of Operating Conditions on the Freeze-Drying of Frozen Particles in a Fixed Bed and Modeling Data. Dry. Technol. 2015, 33, 1892–1898. [Google Scholar] [CrossRef]
- Trelea, I.C.; Passot, S.; Marin, M.; Fonseca, F. Model for Heat and Mass Transfer in Freeze-Drying of Pellets. J. Biomech. Eng. 2009, 131, 1301. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Campos, R.; Fayos-Fernández, J.; Lozano-Guerrero, A.J.; Martínez-González, A.; Monzó-Cabrera, J.; Mediavilla, I.; Peña-Carro, D.; Esteban-Pascual, L.S. Permittivity Measurements for Cypress and Rockrose Biomass Versus Temperature, Density, and Moisture Content. Sensors 2020, 20, 4684. [Google Scholar] [CrossRef] [PubMed]
- Kreuß, M.; Krause, I.; Kulozik, U. Influence of glycosylation on foaming properties of bovine caseinomacropeptide. Int. Dairy J. 2009, 19, 715–720. [Google Scholar] [CrossRef]
- Fischer, K. Neues Verfahrens zur maßanalytischen Bestimmung des Wassergehalts von Flüssigkeiten und festen Körpern. Angew. Chem. 1935, 48, 394–396. [Google Scholar] [CrossRef]
- Bell, L.N.; Labuza, T.P. Moisture Sorption: Practical Aspects of Isotherm Measurement and Use, 2nd ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000; ISBN 1891127187. [Google Scholar]
- Karathanos, V.T.; Villalbobos, G.; Saravacos, G.D. Comparison of Two Methods of Estimation of the Effective Moisture Diffusivity from Drying Data. J. Food Sci. 1990, 55, 218–231. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975; ISBN 0198533446. [Google Scholar]
- Tutuncu, M.A.; Labuza, T.P. Effect of Geometry on the Effective Moisture Transfer Diffusion Coefficient. J. Food Eng. 1996, 30, 443–447. [Google Scholar] [CrossRef]
- Salahi, M.R.; Mohebbi, M.; Taghizadeh, M. Foam-Mat Drying of Cantaloupe (Cucumis melo): Optimization of Foaming Parameters and Investigating Drying Characteristics. J. Food Process. Preserv. 2015, 39, 1798–1808. [Google Scholar] [CrossRef]
- Wilson, A. Foams. Physics, Chemistry and Structure; Springer: London, UK, 1989; ISBN 978-1-4471-3809-9. [Google Scholar]
- Yang, G.; Gilstrap, K.; Zhang, A.; Xu, L.X.; He, X. Collapse temperature of solutions important for lyopreservation of living cells at ambient temperature. Biotechnol. Bioeng. 2010, 106, 247–259. [Google Scholar] [CrossRef]









| Concentration Maltodextrin, % | Freezing Rate, °C∙min−1 | Thickness of Samples, L, cm |
|---|---|---|
| 10 | - | 4.90 |
| 20 | 2.5 | 2.16 |
| 25 | 2.5 | 1.37 |
| 30 | 3.5 | 0.85 |
| 35 | 6.0 | 0.60 |
| 40 | 7.0 | 0.34 |
| Maltodextrin Content, % | 10 | 20 | 30 | 40 |
| Bubble size, d50, µm | 553 ± 51 | 202 ± 29 | 102 ± 14 | 58 ± 10 |
| Overrun, % | 1671 ± 251 | 715 ± 71 | 233 ± 7 | 41 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubbutat, P.; Tauchnitz, A.; Kulozik, U. Water Vapor Pathways during Freeze-Drying of Foamed Product Matrices Stabilized by Maltodextrin at Different Concentrations. Processes 2020, 8, 1463. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8111463
Kubbutat P, Tauchnitz A, Kulozik U. Water Vapor Pathways during Freeze-Drying of Foamed Product Matrices Stabilized by Maltodextrin at Different Concentrations. Processes. 2020; 8(11):1463. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8111463
Chicago/Turabian StyleKubbutat, Peter, Annika Tauchnitz, and Ulrich Kulozik. 2020. "Water Vapor Pathways during Freeze-Drying of Foamed Product Matrices Stabilized by Maltodextrin at Different Concentrations" Processes 8, no. 11: 1463. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8111463