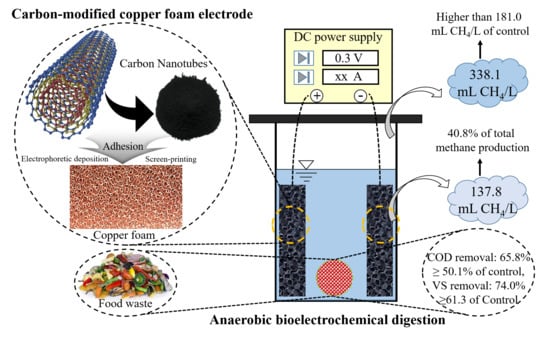
Bioelectrochemical Methane Production from Food Waste in Anaerobic Digestion Using a Carbon-Modified Copper Foam Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Reactor Start-up and Operation
2.3. Analysis and Calculation
3. Results and discussion
3.1. Bioelectrochemical Methane Production
3.2. Process Stability
3.3. Electrochemical Characterization
3.4. Implications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food waste generation and industrial uses: A review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Kibler, K.M.; Reinhart, D.; Hawkins, C. Food waste and the food-energy-water nexus: A review of food waste management alternatives. Waste Manag. 2018, 74, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Palmiotto, M.; Fattore, E.; Paiano, V.; Celeste, G.; Colombo, A.; Davoli, E. Influence of a municipal solid waste landfill in the surrounding environment: Toxicological risk and odor nuisance effects. Environ. Int. 2014, 68, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, H.; Ren, L.; He, L. Experimental and modeling approaches for food waste composting: A review. Chemosphere 2013, 93, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and Kinetic Model of a Single-Stage Anaerobic Digestion System Operated at Different Successive Operating Stages for the Treatment of Food Waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef] [Green Version]
- Dhamodharan, K.; Ajay, S.K. Pre-treatment and anaerobic digestion of food waste for high rate methane production–A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar]
- Capson-Tojo, G.; Rouez, M.; Crest, M. Food waste valorization via anaerobic processes: A review. Rev. Environ. Sci. Bio/Technol. 2016, 15, 499–547. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.; Bae, B. Influence of applied voltage on the performance of bioelectrochemical anaerobic digestion of sewage sludge and planktonic microbial communities at ambient temperature. Bioresour. Technol. 2016, 220, 500–508. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C. Surface modification of a graphite fiber fabric anode for enhanced bioelectrochemical methane production. Energy Fuels 2016, 30, 6467–6474. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.A.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.Q.; Yang, Y.; Sun, G.D.; Wu, D.L. Impact of applied voltage on methane generation and microbial activities in an anaerobic microbial electrolysis cell (MEC). Chem. Eng. J. 2016, 283, 260–265. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, A.J.; Jia, J.N.; Liang, Q.; Liu, Q.; Xing, D.F.; Ren, N.Q. Characterization of methane producti, on and microbial community shifts during waste activated sludge degradation in microbial electrolysis cells. Bioresour. Technol. 2015, 175, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.C.; Ahn, Y. Electroactive microorganisms in bulk solution contribute significantly to methane production in bioelectrochemical anaerobic reactor. Bioresour. Technol. 2018, 259, 119–127. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Bioelectrochemical enhancement of direct interspecies electron transfer in upflow anaerobic digester with effluent recirculation for acidic distillery wastewater. Bioresour. Technol. 2017, 241, 171–180. [Google Scholar] [CrossRef]
- Beegle, J.R.; Abhijeet, P.B. Energy production from waste: Evaluation of anaerobic digestion and bioelectrochemical systems based on energy efficiency and economic factors. Renew. Sustain. Energy Rev. 2018, 96, 343–351. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C. Decoration of graphite fiber fabric cathode with electron transfer assisting material for enhanced bioelectrochemical methane production. J. Appl. Electrochem. 2016, 46, 1211–1219. [Google Scholar] [CrossRef]
- Guo, K.; Prevoteau, A.; Patil, S.A. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–156. [Google Scholar] [CrossRef]
- Guo, K.; Hidalgo, D.; Tommasi, T. Pyrolytic carbon-coated stainless steel felt as a high-performance anode for bioelectrochemical systems. Bioresour. Technol. 2016, 211, 664–668. [Google Scholar] [CrossRef]
- Mook, W.T.; Aroua, M.K.T.; Chakrabarti, M.H. A review on the effect of bio-electrodes on denitrification and organic matter removal processes in bio-electrochemical systems. J. Ind. Eng. Chem. 2013, 19, 1–13. [Google Scholar] [CrossRef]
- Jiang, X.; Lou, S.; Chen, D.; Shen, J.; Han, W.; Sun, X.; Wang, L. Fabrication of polyaniline/graphene oxide composite for graphite felt electrode modification and its performance in the bioelectrochemical system. J. Electroanal. Chem. 2015, 744, 95–100. [Google Scholar] [CrossRef]
- Gooding, J.J.; Gonçales, V.R. Recent advances in the molecular level modification of electrodes for bioelectrochemistry. Curr. Opin. Electrochem. 2017, 5, 203–210. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Influence of neutralization in acidic distillery wastewater on direct interspecies electron transfer for methane production in an upflow anaerobic bioelectrochemical digester. Int. J. Hydrog. Energy 2017, 5, 228. [Google Scholar] [CrossRef]
- Anderson, G.K.; Yang, G. Determination of bicarbonate and total volatile acid concentration in anaerobic digesters using a simple titration. Water Environ Res. 1992, 64, 53–59. [Google Scholar] [CrossRef]
- Choi, K.S.; Sanath, K.; Booki, M. Bioelectrochemical methane (CH4) production in anaerobic digestion at different supplemental voltages. Bioresour. Technol. 2017, 245, 826–832. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wang, X. Effects of alkalinity sources on the stability of anaerobic digestion from food waste. Waste Manag. Res. 2015, 33, 1033–1040. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhang, Y.B.; Wang, L.Y.; Quan, X. Potential for direct interspecies electron transfer in an electricanaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 11094. [Google Scholar] [CrossRef]
- Song, Y.C.; Feng, Q.; Ahn, Y. Performance of the bio-electrochemical anaerobic digestion of sewage sludge at different hydraulic retention times. Energy Fuels 2016, 30, 352–359. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Kim, D.H.; Kim, M.S.; Kim, D.H. Influence of the temperature and hydraulic retention time in bioelectrochemical anaerobic digestion of sewage sludge. Int. J. Hydrog. Energy 2019, 44, 2170–2179. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Liu, C.F.; Yuan, X.Z.; Zeng, G.M.; Li, W.W.; Li, J. Prediction of methane yield at optimum pH for anaerobic digestion of organic fraction of municipal solid waste. Bioresour. Technol. 2008, 99, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.R.; Liu, Y. Chemically Enhanced Primary Sludge as an Anaerobic Co-Digestion Additive for Biogas Production from Food Waste. Processes 2019, 7, 709. [Google Scholar] [CrossRef] [Green Version]
- Koch, C.; Kuchenbuch, A.; Kretzschmar, J.; Wedwitschka, H.; Liebetrau, J.; Muller, S.; Harnisch, F. Coupling electric energy and biogas production in anaerobic digesters—Impacts on the microbiome. RSC Adv. 2015, 5, 31329–31340. [Google Scholar] [CrossRef]
- Chow, W.L.; Chong, S.H.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.M.; Choi, C.H.; Kim, M.; Hyun, M.S.; Shin, S.H.; Yi, D.H.; Kim, H.J. Enrichment of electrochemically active bacteria using a three-electrode electrochemical cell. J. Microbiol. Biotechnol. 2007, 17, 110–115. [Google Scholar]
- Baron, D.; LaBelle, E.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1. J. Biol. Chem. 2009, 284, 28865–28873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Song, Y.C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Polarized electrode enhances biological direct interspecies electron transfer for methane production in upflow anaerobic bioelectrochemical digester. Chemosphere 2018, 204, 186–192. [Google Scholar] [CrossRef]
- Lovley, D.R. Reach out and touch someone: Potential impact of DIET (direct interspecies energy transfer) on anaerobic biogeochemistry, bioremediation, and bioenergy. Rev. Environ. Sci. Biotechnol. 2011, 10, 101–105. [Google Scholar] [CrossRef] [Green Version]





| Parameters | Seeding Sludge | Food Waste | Initial Liquid |
|---|---|---|---|
| pH | 7.02 | 4.46 | 7.2 |
| Alkalinity (mg/L as CaCO3) | 3290 | / | 6000 |
| Chemical oxygen demand (COD, mg/L) | 30,725 | 14,382 | 19,200 |
| Total solid (TS, mg/L) | 26,500 | 15,933 | 16,494 |
| Volatile solid (VS, mg/L) | 12,206 | 10,287 | 11,981 |
| Reactors | ABD | Control | |
|---|---|---|---|
| Lag phase (λ, d) | 2.17 ± 0.12 | 3.08 ± 0.07 | |
| Maximum methane production rate (μm, mL CH4/L.d) | 90.4 ± 3.5 | 53.3 ± 4.3 | |
| Ultimate methane production (Pu, mL CH4/L) | 338.1 ± 6.6 | 181.0 ± 6.5 | |
| λ(d) | 2.17 ± 0.12 | 3.08 ± 0.07 | |
| Adj-r2 | 0.999 | 0.996 | |
| Final methane content (%) | 70.2 ± 3.7 | 48.1 ± 2.7 | |
| Methane yield (mL CH4/g CODr) | 255.3 ± 7.6 | 178.4 ± 6.5 | |
| Current density (A/m2) | Total period | 0.455 ± 0.011 | / |
| Stable period | 0.494 ± 0.010 | ||
| Methane production via electrode (mLCH4/L) | 137.8 ± 20.9 | / | |
| pH | 7.41 ± 0.02 | 7.28 ± 0.04 | |
| Alkalinity (mg/L as CaCO3) | 7405 ± 233 | 6015 ± 451 | |
| COD removal efficiency | 65.8 ± 1.2 | 50.1 ± 1.5 | |
| VS removal efficiency | 74.0 ± 2.2 | 61.3 ± 0.8 | |
| Parameters | ABD | Control | |
|---|---|---|---|
| Oxidation current (mA) | 0.42 | 0.15 | |
| Oxidation potential (V vs. Ag/AgCl) | 0.36 | 0.35 | |
| Reduction current (mA) | 0.15 | Not detected | |
| Reduction potential (V vs. Ag/AgCl) | −0.53 | Not detected | |
| Electrode current density (A/m2) | Start-up period | 0.323 ± 0.024 | / |
| Sable period | 0.494 ± 0.010 | / | |
| Final period | 0.220 ± 0.009 | / | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Z.; Feng, Q.; Zhao, R.; Wang, X. Bioelectrochemical Methane Production from Food Waste in Anaerobic Digestion Using a Carbon-Modified Copper Foam Electrode. Processes 2020, 8, 416. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8040416
An Z, Feng Q, Zhao R, Wang X. Bioelectrochemical Methane Production from Food Waste in Anaerobic Digestion Using a Carbon-Modified Copper Foam Electrode. Processes. 2020; 8(4):416. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8040416
Chicago/Turabian StyleAn, Zhengkai, Qing Feng, Rusong Zhao, and Xiaoli Wang. 2020. "Bioelectrochemical Methane Production from Food Waste in Anaerobic Digestion Using a Carbon-Modified Copper Foam Electrode" Processes 8, no. 4: 416. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8040416