Performance and Kinetics of Bioaugmentation, Biostimulation, and Natural Attenuation Processes for Bioremediation of Crude Oil-Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Configuration of the Tanks
2.3. Loading of the Tanks
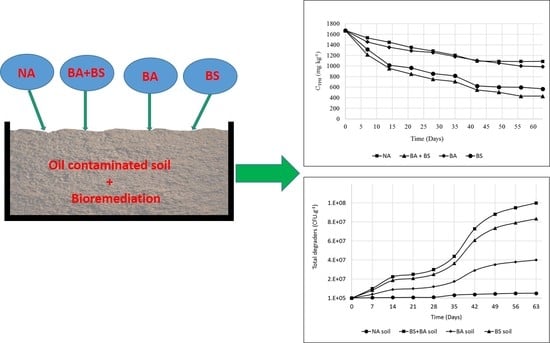
2.4. Natural Attenuation (Tank 1)
2.5. Bioaugmentation + Biostimulation (Tank 2)
2.6. Bioaugmentation (Tank 3)
2.7. Biostimulation (Tank 4)
2.8. Oxygen Supply and Moisture Content
2.9. Soil Analyses
2.9.1. TPH Analysis
2.9.2. pH Analysis
2.9.3. Moisture Content
2.9.4. Organic Matter
2.9.5. Organic Carbon
2.9.6. Nutrients
2.10. Microbial Analysis
2.11. Kinetic Model
- r: reaction rate,
- k: biodegradation rate,
- C: concentration,
- t: time,
- n: reaction order.
- µ: specific growth rate,
- µmax: maximum specific growth rate,
- Ks: TPH value at half-time.
3. Results
3.1. Changes in Soil TPH Concentrations
3.2. Microbial Analysis
3.3. Bioremediation Kinetics
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Abed, R.M.; Al-Sabahi, J.; Al-Maqrashi, F.; Al-Habsi, A.; Al-Hinai, M. Characterization of hydrocarbon-degrading bacteria isolated from oil-contaminated sediments in the sultanate of Oman and evaluation of bioaugmentation and biostimulation approaches in microcosm experiments. Int. Biodeterior. Biodegrad. 2014, 89, 58–66. [Google Scholar] [CrossRef]
- Cerqueira, V.S.; Peralba, M.D.C.R.; Camargo, F.A.D.O.; Bento, F.M. Comparison of bioremediation strategies for soil impacted with petrochemical oily sludge. Int. Biodeterior. Biodegrad. 2014, 95, 338–345. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V. Effect of remediation strategies on biological activity of oil-contaminated soil—A field study. Int. Biodeterior. Biodegrad. 2018, 126, 57–68. [Google Scholar] [CrossRef]
- Jiang, Y.; Brassington, K.J.; Prpich, G.; Paton, G.I.; Semple, K.T.; Pollard, S.J.; Coulon, F. Insights into the biodegradation of weathered hydrocarbons in contaminated soils by bioaugmentation and nutrient stimulation. Chemosphere 2016, 161, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Bioavailability of weathered hydrocarbons in engine oil-contaminated soil: Impact of bioaugmentation mediated by pseudomonas spp. on bioremediation. Sci. Total. Environ. 2018, 636, 968–974. [Google Scholar] [CrossRef]
- Safdari, M.-S.; Kariminia, H.-R.; Rahmati, M.; Fazlollahi, F.; Polasko, A.; Mahendra, S.; Wilding, W.V.; Fletcher, T.H. Development of bioreactors for comparative Study of natural attenuation, biostimulation, and bioaugmentation of petroleum-hydrocarbon contaminated Soil. J. Hazard Mater. 2018, 342, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Hechmi, N.; Bosso, L.; El-Bassi, L.; Scelza, R.; Testa, A.; Jedidi, N.A.; Rao, M. Depletion of pentachlorophenol in soil microcosms with byssochlamys nivea and scopulariopsis brumptii as detoxification agents. Chemosphere 2016, 165, 547–554. [Google Scholar] [CrossRef]
- Tian, H.; Yan, M.; Treu, L.; Angelidaki, I.; Fotidis, I.A. Hydrogenotrophic methanogens are the key for a successful bioaugmentation to alleviate ammonia inhibition in thermophilic anaerobic Digesters. Bioresour. Technol. 2019, 293, 122070. [Google Scholar] [CrossRef]
- Łebkowska, M.; Zborowska, E.; Karwowska, E.; Miaśkiewicz-Pęska, E.; Muszyński, A.; Tabernacka, A.; Naumczyk, J.; Jęczalik, M. Bioremediation of soil polluted with fuels by sequential multiple injection of native microorganisms: Field-scale processes in Poland. Ecol. Eng. 2011, 37, 1895–1900. [Google Scholar] [CrossRef]
- Taccari, M.; Milanovic, V.; Comitini, F.; Casucci, C.; Ciani, M. Effects of biostimulation and bioaugmentation on diesel removal and bacterial community. Int. Biodeterior. Biodegrad. 2012, 66, 39–46. [Google Scholar] [CrossRef]
- Wu, M.; Chen, L.; Tian, Y.; Ding, Y.; Dick, W.A. Degradation of polycyclic aromatic hydrocarbons by microbial consortia enriched from three soils using two different culture media. Environ. Pollut. 2013, 178, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wong, A.; Yau, K.; Wong, Y.; Tam, N.F. Natural attenuation, biostimulation and bioaugmentation on biodegradation of polycyclic aromatic hydrocarbons (PAHs) in mangrove sediments. Mar. Pollut. Bull. 2005, 51, 1071–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppi, S.; Sinkkonen, A.; Romantschuk, M. Enhancing bioremediation of diesel-fuel-contaminated soil in a boreal climate: Comparison of biostimulation and bioaugmentation. Int. Biodeterior. Biodegrad. 2011, 65, 359–368. [Google Scholar] [CrossRef]
- Sayara, T.; Borràs, E.; Caminal, G.; Sarrà, M.; Sánchez, A. Bioremediation of PAHs-contaminated soil through composting: Influence of bioaugmentation and biostimulation on contaminant biodegradation. Int. Biodeterior. Biodegrad. 2011, 65, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Balba, M.; Al-Awadhi, N.; Al-Daher, R. Bioremediation of oil-Contaminated soil: Microbiological methods for feasibility assessment and field evaluation. J. Microbiol. Methods 1998, 32, 155–164. [Google Scholar] [CrossRef]
- Wu, M.; Ye, X.; Chen, K.; Li, W.; Yuan, J.; Jiang, X. Bacterial community shift and hydrocarbon transformation during bioremediation of short-term petroleum-contaminated soil. Environ. Pollut. 2017, 223, 657–664. [Google Scholar] [CrossRef]
- Wu, M.; Dick, W.A.; Li, W.; Wang, X.C.; Yang, Q.; Wang, T.; Xu, L.; Zhang, M.; Chen, L. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int. Biodeterior. Biodegrad. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Nwankwegu, A.S.; Onwosi, C.O. Bioremediation of gasoline contaminated agricultural soil by bioaugmentation. Environ. Technol. Innov. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Alexander, M. Biodegradation and Bioremediation; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Zhang, X.-X.; Cheng, S.; Zhu, C.-J.; Sun, S.-L. Microbial PAH-degradation in soil: Degradation pathways and contributing factors. Pedosphere 2006, 16, 555–565. [Google Scholar] [CrossRef]
- Alavi, N.; Mesdaghinia, A.; Naddafi, K.; Mohebali, G.; Daraei, H.; Maleki, A.; Alaei, L. Biodegradation of petroleum hydrocarbons in a soil polluted sample by oil-based drilling cuttings. Soil Sediment Contam. Int. J. 2014, 23, 586–597. [Google Scholar] [CrossRef]
- USEPA. Test Methods for Evaluating Solid Waste, Physical/Chemical Methods SW-846; EPA Publication: Washington, DC, USA, 2015. [Google Scholar]
- USEPA. Hexadecane Extraction and Screening of Purgeable Organics; USEPA: Washington, DC, USA, 1986.
- USEPA. Method 8015B Nonhalogenated Organics Using GC/FID; USEPA: Washington, DC, USA, 2007.
- USEPA. Method 9045D Soil and Waste pH; USEPA: Washington, DC, USA, 2004.
- Shin, H.; Yu, J.; Wang, L.; Jeong, Y.; Kim, J. Spectral interference of heavy metal contamination on spectral signals of moisture content for heavy metal contaminated soils. IEEE Trans. Geosci. Remote. Sens. 2020, 58, 2266–2275. [Google Scholar] [CrossRef]
- Bosso, L.; Scelza, R.; Testa, A.; Cristinzio, G.; Rao, M. Depletion of pentachlorophenol contamination in an agricultural soil treated with byssochlamys nivea, scopulariopsis brumptii and urban waste compost: A laboratory microcosm study. Water Air Soil Pollut. 2015, 226, 183. [Google Scholar] [CrossRef]
- ASTM. ASTM D2974-14 Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Xue, W.; Peng, Z.; Huang, D.-L.; Zeng, G.; Wan, J.; Xu, R.; Cheng, M.; Zhang, C.; Jiang, D.; Hu, Z. Nanoremediation of cadmium contaminated river sediments: Microbial response and organic carbon changes. J. Hazard Mater. 2018, 359, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bernard, B.B.; Bernard, H.; Brooks, J.M. Determination of TC, TOC, and TIC in Sediments; TDI Brooks International: College Station, TX, USA, 2004. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis; ISRIC: Wageningen, The Netherlands, 2002. [Google Scholar]
- Li, J.; Gong, J.; Fu, B.; Huang, Z.; Huang, Y.; Gui, L. Effect of land use conversion on soil organic carbon sequestration in the loess hilly area, loess plateau of China. Ecol. Res. 2006, 22, 641–648. [Google Scholar]
- Klaic, P.M.A.; Nunes, A.M.; Moreira, A.D.S.; Vendruscolo, C.; Ribeiro, A.S. Determination of Na, K, Ca and Mg in Xanthan gum: Sample treatment by acid digestion. Carbohydr. Polym. 2011, 83, 1895–1900. [Google Scholar] [CrossRef]
- Nakasaki, K.; Hirai, H. Temperature control strategy to enhance the activity of yeast inoculated into compost raw material for accelerated composting. Waste Manag. 2017, 65, 29–36. [Google Scholar] [CrossRef]
- Komilis, D.; Vrohidou, A.-E.K.; Voudrias, E.A. Kinetics of aerobic bioremediation of a diesel-contaminated sandy soil: Effect of nitrogen addition. Water Air Soil Pollut. 2009, 208, 193–208. [Google Scholar] [CrossRef]
- Chemlal, R.; Tassist, A.; Drouiche, M.; Lounici, H.; Mameri, N.; Drouiche, N. Microbiological aspects study of bioremediation of diesel-contaminated soils by biopile technique. Int. Biodeterior. Biodegrad. 2012, 75, 201–206. [Google Scholar] [CrossRef]
- Tellez, G.T.; Nirmalakhandan, N.; Gardeatorresdey, J.L. Evaluation of biokinetic coefficients in degradation of oil-field produced water under varying salt concentrations. Water Res. 1995, 29, 1711–1718. [Google Scholar] [CrossRef]
- Eweis, J.B.; Ergas, S.J.; Chang, D.P.Y.; Schroeder, E.D. Bioremediation Principles; McGraw-Hill Book Company Europe: Maidenhead, UK, 1998; p. 296. [Google Scholar]
- Coulon, F.; Brassington, K.J.; Bazin, R.; Linnet, P.E.; Thomas, K.A.; Mitchell, T.R.; Lethbridge, G.; Smith, J.W.N.; Pollard, S.J. Effect of fertilizer formulation and bioaugmentation on biodegradation and leaching of crude oils and refined products in soils. Environ. Technol. 2012, 33, 1879–1893. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Kechavarzi, C.; Li, X.; Sui, H.; Pollard, S.J.; Coulon, F. Influence of mature compost amendment on total and bioavailable polycyclic aromatic hydrocarbons in contaminated soils. Chemosphere 2013, 90, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, E.; Adetutu, E.M.; Anderson, P.; Ball, A.S. Necrophytoremediation of phenanthrene and pyrene in contaminated soil. J. Environ. Manag. 2013, 122, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Brook, T.R.; Stiver, W.H.; Zytner, R.G. Biodegradation of diesel fuel in soil under various nitrogen addition regimes. Soil Sediment Contam. Int. J. 2001, 10, 539–553. [Google Scholar] [CrossRef]
- Shewfelt, K.; Lee, H.; Zytner, R.G. Optimization of Nitrogen for Bioventing of Gasoline Contaminated Soil. J. Environ. Eng. Sci. 2005, 4, 29–42. [Google Scholar] [CrossRef]
- Rončević, S.; Dalmacija, B.; Ivancev-Tumbas, I.; Tričković, J.; Petrovic, O.; Klasnja, M.; Agbaba, J. Kinetics of degradation of hydrocarbons in the contaminated soil layer. Arch. Environ. Contam. Toxicol. 2005, 49, 27–36. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Kravchenko, I.; Xu, H.; Zhang, C.-G. Dynamic changes in microbial activity and community structure during biodegradation of petroleum compounds: A Laboratory experiment. J. Environ. Sci. 2007, 19, 1003–1013. [Google Scholar] [CrossRef]
- Mancera-López, M.; Esparza-García, F.; Chávez-Gómez, B.; Rodríguez-Vázquez, R.; Saucedo-Castañeda, G.; Barrera-Cortés, J. Bioremediation of an aged hydrocarbon-contaminated soil by a combined System of biostimulation–bioaugmentation with filamentous fungi. Int. Biodeterior. Biodegrad. 2008, 61, 151–160. [Google Scholar] [CrossRef]
- Suja, F.; Rahim, F.; Taha, M.R.; Hambali, N.; Razali, M.R.; Khalid, A.; Hamzah, A. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeterior. Biodegrad. 2014, 90, 115–122. [Google Scholar] [CrossRef]
- Smith, M.J.; Lethbridge, G.; Burns, R.G. Fate of phenanthrene, pyrene and benzo[a]pyrene during biodegradation of crude oil added to two soils. FEMS Microbiol. Lett. 1999, 173, 445–452. [Google Scholar] [CrossRef]
- Krutz, L.J.; Beyrouty, C.A.; Gentry, T.J.; Wolf, D.C.; Reynolds, C.M. Selective enrichment of a pyrene degrader population and enhanced pyrene degradation in Bermuda grass rhizosphere. Biol. Fertil. Soils 2005, 41, 359–364. [Google Scholar] [CrossRef]





| Microbial Inoculum Addition (Bioaugmentation) | Nutrients Addition (Biostimulation) (%20 N, %10 P, %10 K) | Crude Oil Addition | |
|---|---|---|---|
| Tank 1 (NA) | No | No | Yes |
| Tank 2 (BS + BA) | Yes | Yes | Yes |
| Tank 3 (BA) | Yes | No | Yes |
| Tank 4 (BS) | No | Yes | Yes |
| Parameters | Unit | Values |
|---|---|---|
| pH | 7.8 ± 0.11 | |
| Total Petroleum Hydrocarbon | mg/kg | 1674 ± 9.25 |
| Organic matter content of soil (foc) | % | 3 ± 0.31 |
| Organic matter | g/kg | 3.20 ± 0.25 |
| Organic carbon | g/kg | 1.86 ± 0.15 |
| Total Nitrogen | mg/kg | 0.002 ± 0.0 |
| C/N ratio (adjusted) | 20 ± 0.36 | |
| Total phosphorous | mg/kg | 0.0005 ± 0.0 |
| Potassium | mg/kg | 0.0005 ± 0.0 |
| Moisture content | % | 10 ± 0.12 |
| Treatment Type | First Order Model | Monod Model |
|---|---|---|
| NA | k:0.0159 d−1 | µmax:0.00875 d−1 |
| t1/2:43.6 d | Ks:1114 mg kg−1 | |
| R2:0.86 | R2:0.90 | |
| BA+BS | k:0.0419 d−1 | µmax:0.02675 d−1 |
| t1/2:16.54 d | Ks:918 mg kg−1 | |
| R2:0.89 | R2:0.97 | |
| BA | k:0.033 d−1 | µmax:0.0104 d−1 |
| t1/2:21.0 d | Ks:1289 mg kg−1 | |
| R2:0.88 | R2:0.89 | |
| BS | k:0.0333 d−1 | µmax:0.0193 d−1 |
| t1/2:20.8 d | Ks:965 mg kg−1 | |
| R2:0.84 | R2:0.90 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaman, C. Performance and Kinetics of Bioaugmentation, Biostimulation, and Natural Attenuation Processes for Bioremediation of Crude Oil-Contaminated Soils. Processes 2020, 8, 883. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8080883
Yaman C. Performance and Kinetics of Bioaugmentation, Biostimulation, and Natural Attenuation Processes for Bioremediation of Crude Oil-Contaminated Soils. Processes. 2020; 8(8):883. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8080883
Chicago/Turabian StyleYaman, Cevat. 2020. "Performance and Kinetics of Bioaugmentation, Biostimulation, and Natural Attenuation Processes for Bioremediation of Crude Oil-Contaminated Soils" Processes 8, no. 8: 883. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8080883