Numerical Study of the Filling Process of a Liquid Hydrogen Storage Tank under Different Sloshing Conditions
Abstract
:1. Introduction
2. Model Description and Numerical Method
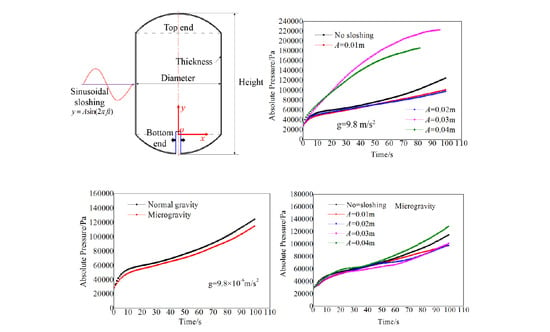
2.1. Physical and Computational Models
2.2. Mathematical Description
2.3. Interfacial Phase Change Model
2.4. Numerical Method and Boundary Condition
3. Model Validation
3.1. Grid Independence
3.2. Verification of the Sloshing Model
3.2.1. Rectangle Tank without Phase Change
3.2.2. Cylindrical Tank with Phase Change
3.2.3. Verification of the Phase Change Model in the Filling Process
4. Results
4.1. Effects of Frequency on the Pressure Curve During the Filling Process
4.2. Effects of Amplitude on the Pressure Curve during the Filling Process
4.3. Effect of Sloshing on Phase Change and Temperature Distribution
4.4. Pressure Response under Microgravity and Sloshing Conditions
5. Discussion
6. Conclusions
- (1)
- Under normal gravity, the increase of frequency reduces the pressure curve and weakens the evaporation process of the liquid when the amplitude is low. However, at high amplitude, the pressure curve increases significantly with the increase in frequency.
- (2)
- Within the scope of this study, there is a critical condition (amplitude A = 0.03 m and frequency f = 3 Hz) for the effect of the sloshing conditions on the pressure curve under normal gravity. When sloshing exceeds the critical condition, the pressure curve of the liquid hydrogen storage tank during the filling process increases. That is, the pressure is higher and increases more quickly during the same time compared to the condition without sloshing. By contrast, when the sloshing is within the critical condition, properly increasing the sloshing causes the storage tank pressure curve to decrease.
- (3)
- Within the scope of this study, the pressure curve under microgravity changes less than normal gravity, even if the amplitude and frequency increase. The sloshing makes it easier for the liquid to spread along the wall during the filling process. This also further weakens the temperature stratification in the storage tank.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | Amplitude (m) |
| E | internal energy (J) |
| Fv | volume force (N∙m−3) |
| P | Pressure (Pa) |
| T | Temperature (K) |
| cp | specific heat at constant pressure (J·kg−1·K−1) |
| f | excitation frequency (Hz) |
| hlg | enthalpy of vaporization (J∙kg−1) |
| v | Velocity (m∙s−1) |
| θ | volume fraction |
| ρ | Density (kg∙m−3) |
| η | Viscosity (Pa∙s) |
| λ | thermal conductivity (W∙m−1∙K−1) |
| σlg | surface tension of gas-liquid interface (N∙m−1) |
| к | surface curvature (m−1) |
| τ | Time (s) |
| Subscripts | |
| g | gas phase |
| l | liquid phase |
| sat | saturation state |
References
- Abramson, H.N. The Dynamic Behavior of Liquids in Moving Containers, with Applications to Space Vehicle Technology; NASA Technical Reports SP-106; NASA: Washington, DC, USA, 1 January 1966.
- Moran, M.E.; McNelis, N.B.; Kudlac, M.T. Experimental results of hydrogen sloshing in a 62 cubic foot (1750 Liter) tank. In Proceedings of the 30th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, AIAA, Indianapolis, IN, USA, 1 June 1994; pp. 1994–3259. [Google Scholar]
- Hopfinger, E.J.; Das, S.P. Mass Transfer Enhancement by Capillary Waves at a Liquid–vapour Interface. Exp. Fluids 2009, 46, 597–605. [Google Scholar] [CrossRef]
- Arndt, T. Sloshing of Cryogenic Liquids in a Cylindrical Tank under Normal Gravity Conditions. Ph.D. Thesis, Center of Applied Space Technology and Microgravity, University of Bremen, Bremen, Germany, 2012. [Google Scholar]
- Ludwig, C.; Dreyer, M.E.; Hopfinger, E.J. Pressure variations in a cryogenic liquid storage tank subjected to periodic excitations. Int. J. Heat Mass Transf. 2013, 66, 223–234. [Google Scholar] [CrossRef]
- Xue, M.A.; Zheng, J.; Lin, P.; Yuan, X. Experimental study on vertical baffles of different configurations in suppressing sloshing pressure. Ocean Eng. 2017, 136, 178–189. [Google Scholar] [CrossRef]
- Agawane, G.; Jadon, V.; Balide, V.; Banerjee, R. An Experimental Study of Sloshing Noise in a Partially Filled Rectangular Tank. SAE Int. J. Passeng. Cars Mech. Syst. 2017, 10, 391–400. [Google Scholar] [CrossRef]
- He, Y.; Zeng, Z.; Yu, Y.; Ruan, D. Liquid fuel sloshing control of an automotive fuel tank. Noise Vib. Worldw. 2019, 50, 227–236. [Google Scholar] [CrossRef]
- Ming, P.J.; Duan, W.Y. Numerical simulation of sloshing in rectangular tank with VOF based on unstructured grids. J. Hydrodyn. 2010, 22, 856–864. [Google Scholar] [CrossRef]
- Konopka, M.; Nöding, P.; Klatte, J.; Behruzi, P.; Gerstmann, J.; Stark, A.; Darkow, N. Analysis of LN2 Filling, Draining, Stratification and Sloshing Experiments. In Proceedings of the AIAA Fluid Dynamics Conference, Washington, DC, USA, 10 June 2016. [Google Scholar]
- Sanapala, V.S.; Velusamy, K.; Patnaik, B.S.V. CFD simulations on the dynamics of liquid sloshing and its control in a storage tank for spent fuel applications. Ann. Nucl. Energy 2016, 94, 494–509. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Lei, G.; Li, Y. Fluid thermal stratification in a non-isothermal liquid hydrogen tank under sloshing excitation. Int. J. Hydrogen Energy 2018, 43, 22622–22635. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Lei, G.; Li, Y. Fluid sloshing dynamic performance in a liquid hydrogen tank. Int. J. Hydrogen Energy 2019, 44, 13885–13894. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Lei, G.; Li, Y. Hydrodynamic performance in a sloshing liquid oxygen tank under different initial liquid filling levels. Aerospace Sci. Technol. 2019, 85, 544–555. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Lei, G.; Li, Y. Sloshing hydrodynamic performance in cryogenic liquid oxygen tanks under different amplitudes. Appl. Therm. Eng. 2019, 150, 359–371. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, Y.; Lei, G.; Li, Y. Sloshing behavior under different initial liquid temperatures in a cryogenic fuel tank. J. Low Temp. Phys. 2019, 196, 347–363. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, H.; Feng, Y.; Liu, Y.; Li, Y. Thermodynamic characteristic in a cryogenic storage tank under an intermittent sloshing excitation. Inter. J. Hydrogen Energy 2020, 45, 12082–12094. [Google Scholar] [CrossRef]
- Dai, J.; Han, M.; Ang, K.K. Moving element analysis of partially filled freight trains subject to abrupt braking. Int. J. Mech. Sci. 2019, 151, 85–94. [Google Scholar] [CrossRef]
- Kolaei, A.; Rakheja, S.; Richard, M.J. Three-dimensional dynamic liquid slosh in partially-filled horizontal tanks subject to simultaneous longitudinal and lateral excitations. Eur. J. Mech. B Fluids 2015, 93, 251–263. [Google Scholar] [CrossRef]
- Moran, M.E.; Nyland, T.W.; Driscoll, S.L. Hydrogen No-Vent Fill Testing in a 1.2 Cubic Foot (34 Liter) Tank; NASA STI/Recon Technical Report N; NASA: Washington, DC, USA, 1 October 1991.
- Ma, Y.; Li, Y.; Zhu, K.; Wang, Y.; Wang, L.; Tan, H. Investigation on no-vent filling process of liquid hydrogen tank under microgravity condition. Int. J. Hydrogen Energy 2017, 42, 8264–8277. [Google Scholar] [CrossRef]
- Kim, K.J.; Jin, L.; Kim, Y.; Jeong, S. Experimental Investigation on No Vent Fill Process of Cryogenic Liquid. In Proceedings of the International Ocean & Polar Engineering Conference, San Francisco, CA, USA, 25–30 June 2017. [Google Scholar]
- Kim, K.J.; Jin, L.; Jeong, S.; Yang, H.S.; Yoo, J.; Choi, Y.S. Experimental and numerical investigation on the possibility of no-vent filling of liquefied natural gas. Cryogenics 2019, 102, 35–44. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, C.; Park, J.; Seo, M.; Jeong, S. Experimental investigation on no-vent fill process using tetrafluoromethane (CF4). Cryogenics 2016, 74, 123–130. [Google Scholar] [CrossRef]
- Kang, Z.; Yanzhong, L.; Yuan, M.; Lei, W.; Fushou, X.; Jiaojiao, W. Experimental study on cool down characteristics and thermal stress of cryogenic tank during LN2 filling process. Appl. Therm. Eng. 2018, 130, 951–961. [Google Scholar] [CrossRef]
- William, M.; Cirillo, W.; Stromgren, C.; Cates, G. Risk analysis of on-orbit spacecraft refueling concepts. In Proceedings of the AIAA Space 2010 Conference & Exposition, Anaheim, CA, USA, 30 August 2010. [Google Scholar]
- Chen, L.; Liu, J.; Lin, X.; Yao, C.; Zhu, H. Numerical simulation of the vane type tank and system on-orbit refueling process. J. Phys. Conf. Ser. 2018, 1074, 012060. [Google Scholar] [CrossRef]
- Cui, C.; Si, P.; Wang, H.; Yan, W.; Li, W.; Sun, J. Numerical Study on Gas-liquidSeparation Performance of Spiral Pipe Used in On-orbit Refueling. In Proceedings of the 2013 International Conference on Mechatronic Sciences, Electric Engineering and Computer (MEC), Shenyang, China, 20–22 December 2013. [Google Scholar]
- Zhai, G.; Zheng, H.M.; Bin, L. Attitude Dynamics of Spacecraft with Time-Varying Inertia during On-Orbit Refueling. J. Guid. Control Dyn. 2017, 41, 1744–1754. [Google Scholar]
- Fu, J.; Sunden, B.; Chen, X. Influence of wall ribs on the thermal stratification and self-pressurization in a cryogenic liquid tank. Appl. Therm. Eng. 2014, 73, 1421–1431. [Google Scholar] [CrossRef]
- Ren, J.J.; Shi, J.Y.; Liu, P.; Bi, M.S.; Jia, K. Simulation on thermal stratification and de-stratification in liquefied gas tanks. Int. J. Hydrogen Energy 2013, 38, 4017–4023. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.Z.; Zhu, K.; Jin, Y.H. Comparison of three computational models for predicting pressurization characteristics of cryogenic tank during discharge. Cryogenics 2015, 65, 16–25. [Google Scholar]
- Melideo, D.; Baraldi, D. CFD analysis of fast filling strategies for hydrogen tanks and their effects on key-parameters. Int. J. Hydrogen Energy 2015, 40, 735–745. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.Z.; Li, C.; Zhao, Z.X. CFD investigation of thermal and pressurization performance in LH2 tank during discharge. Cryogenics 2013, 57, 63–73. [Google Scholar] [CrossRef]
- Lee, H.; Kharangate, C.R.; Mascarenhas, N.; Park, I.; Mudawar, I. Experimental and computational investigation of vertical downflow condensation. Int. J. Heat Mass Tran. 2015, 85, 865–879. [Google Scholar] [CrossRef]
- Fluent, A. ANSYS Fluent Theory Guide; ANSYS Inc.: Canonsburg, PA, USA, 2017; Available online: http://www.ansys.com (accessed on 10 July 2020).
- Available online: https://www.nist.gov/srd/refprop (accessed on 13 September 2019).
- Available online: https://www.matdat.com/ (accessed on 10 July 2020).
- Manuel, H.; Stefan, A.; Roger, F. Computational modeling of nonlinear propellant sloshing for spacecraft AOCS applications. Ceas Space J. 2018, 10, 441–451. [Google Scholar]
- Grotle, E.L.; Æsøy, V. Dynamic modelling of the thermal response enhanced by sloshing in marine LNG fuel tanks. Appl. Therm. Eng. 2018, 135, 512–520. [Google Scholar] [CrossRef]
- Kosowski, K.; Tucki, K.; Kosowski, A. Application of Artificial Neural Networks in Investigations of Steam Turbine Cascades. J. Turbomach. 2010, 135, 014501–014505. [Google Scholar] [CrossRef]




















| Properties | Vapor | Liquid | Solid |
|---|---|---|---|
| cp/J·kg−1·K−1 | 14274.27 − 0.366T + 0.00161T2 − 4.630 × 10−7T3 + 4.103 × 10−11T4 | 5.657 × 105 + 1.459 × 105T − 1.471 × 104T2 + 7.322 × 102T3 − 17.98T4 + 0.175T5 | −4.7335 + 0.848T |
| /W·m−1·K−1 | 0.0827 + 0.000356T + 1.072 × 10−8T2 − 4.405 × 10−12T3 + 3.263 × 10−16T4 | 7.267 × 10−2 − 1.294 × 10-3T + 5.948 × 10−4T2 − 3.969 × 10-5T3 + 1.081 × 10−6T4 − 1.147 × 10−8T5 | 2.839 − 0.1952T + 0.007T2 |
| /Pa·s | 3.773 × 10−6 + 1.932 × 10−8T − 3.231 × 10−12T2 + 4.917 × 10−16T3 − 3.024 × 10−20T4 | 1.537 × 10−4 − 1.868 × 10−5T + 9.596 × 10−7T2 − 2.290 × 10−8T3 + 2.071 × 10−10T4 | |
| /kg·m−3 | 50.137 + 6.9105T − 0.5756T2 + 0.0191T3 − 0.0002T4 | 8300 |
| Temperature/K | Absolute Pressure/Pa | Vapor Volume Fraction | |
|---|---|---|---|
| Wall | T = 44.3 K, τ = 0, | ||
| Inlet | T = Tsat, τ > 0, | P = psat, τ > 0, | 0, τ > 0, |
| Fluid | T = Tsat, τ = 0, | P = 27,597 Pa, τ = 0, | 1, τ = 0, |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Zhang, J. Numerical Study of the Filling Process of a Liquid Hydrogen Storage Tank under Different Sloshing Conditions. Processes 2020, 8, 1020. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8091020
Wei G, Zhang J. Numerical Study of the Filling Process of a Liquid Hydrogen Storage Tank under Different Sloshing Conditions. Processes. 2020; 8(9):1020. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8091020
Chicago/Turabian StyleWei, Guomeng, and Jianfei Zhang. 2020. "Numerical Study of the Filling Process of a Liquid Hydrogen Storage Tank under Different Sloshing Conditions" Processes 8, no. 9: 1020. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8091020