Chitosan Plasma Chemical Processing in Beam-Plasma Reactors as a Way of Environmentally Friendly Phytostimulants Production
Abstract
:1. Introduction
- To upgrade the experimental setup available and adapt its operations to obtain low molecular weight chitosan oligomers in amounts sufficient for practical agricultural uses, i.e., to develop the industrial Electron-Beam Plasma Chemical Reactor (EBPR) prototype;
- To characterize properties of COS obtained by chitosan degradation under beam-plasma action in the EBPR;
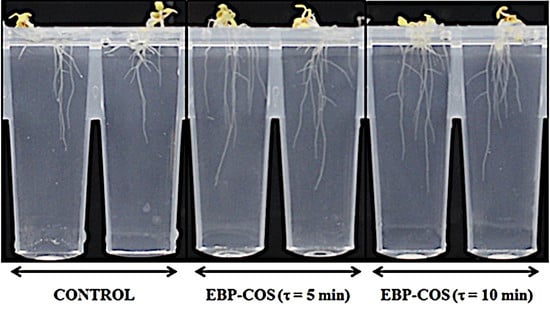
- To study the COS ability to stimulate important plant productivity parameters (root length, number of flowers, and fruits).
2. Materials and Methods
2.1. The Electron-Beam Plasma Chemical Reactor for Chitosan Processing and Treatment Procedure
2.2. Characterization of the EBP-Treated Chitosan
2.2.1. Solubility Measurements
2.2.2. Molecular Weight Measurement
2.2.3. Transform Infrared Spectroscopy (FT-IR)
2.2.4. Deacetylation Degree Measurement
2.3. Bio-Activity of the EBP-Produced COS: Plant Experiments
2.3.1. Influence of the EBP-Produced COS on the Root Growth
2.3.2. Greenhouse Trial: Biostimulating Effects of the EBP-Produced COS on Tomato Plants
2.4. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the EBP-Produced COS
3.2. Plant Bioactivity of the EBP-Produced COS
3.3. Possible Mechanisms of the EBP-Action on Chitosan
- Chemically active heavy particles of the EBP: excited molecules and atoms, ions, radicals, e.g., active oxygen species;
- Fast electrons of the partially degraded EB that bombard material;
- Secondary electrons of moderate energies (0.01–1 keV) produced in the EBP due to ionization of the plasma generating media molecules;
- The EBP-radiation, especially UV and X-ray (bremsstrahlung);
- Possible heating by direct electron bombardment and due to heat transfer between the plasma cloud and sample.
3.4. Advantages and Power Efficiency of the COS Production in EBP-Assisted Processes in Comparison with Conventional Methods
- Efficiency of the electric power conversion into the electron beam power;
- Power loss in the injection window;
- Utilization efficiency of the injected EB, i.e., the ratio of the power directly used in material treatment to the total power of the EB injected into the reaction chamber.
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Beam-Plasma Generator Part | Unit Version | Weight, kg | Product Manufacturer | Maintenance |
|---|---|---|---|---|
| Electron gun | Thermionic cathode LaB6 | ≈10 | Numerous manufacturers of welding electron guns, e.g., PROGRESS Company, Russia | Lifetime more than 100 h under normal conditions |
| Plasma cathode | ≈8 | TUSUR University, Russia | Unlimited lifetime | |
| High voltage power source | High voltage generators of positive or negative polarity | ≈15 | Spellman High Voltage Electronics Corporation, USA | No maintenance |
| Beam control units | Controller of the beam power (semi-automatic) | ≈20 | TUSUR University, Russia | No maintenance |
| Controllers of the beam power and beam scanning (automatic) | ≈25 | TUSUR University, Russia | No maintenance | |
| Injection window | Gas-dynamic window | ≈2 | Moscow Institute of Physics and Technology, Russia | Lifetime about 100 h |
| Gas-dynamic window with a differential pumping stage | ≈5 | Moscow Institute of Physics and Technology, Russia | Lifetime about 200 h | |
| High vacuum system | Diffusion pump in combination with oil sealed pump | ≈35 | VACMA, Russia | Minimal maintenance |
| Turbo molecular pump with dry scroll pump | ≈35 | Numerous manufacturers, e.g., STP Maglev with XDS, Edwards Vacuum, UK | No maintenance | |
| Total weight | 90–100 |
References
- Olicon-Hernandez, D.R.; Zepeda-Giraud, L.F.; Guerra-Sanchez, G. Current applications of chitosan and chitooligonaccharides. A review. J. Drug Des. Res. 2017, 4, 1039. [Google Scholar]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Xing, R.; Liu, Y.; Li, K.; Yu, H.; Liu, S.; Yang, Y.; Chen, X.; Li, P. Monomer composition of chitooligosaccharides obtained by different degradation methods and their effects on immunomodulatory activities. Carbohydr. Polym. 2017, 157, 1288–1297. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Guilger, M.; Pascoli, M.; Bileshy-Jose, N.; Abhilash, P.C.; Fraceto, L.F.; de Lima, R. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 2016, 6, 19768. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Malek, M.A.; Puteh, A.B.; Ismail, M.R.; Ashrafuzzaman, M.; Naher, L. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop Sci. 2012, 6, 918–921. [Google Scholar]
- Chatelain, P.G.; Pintado, M.E.; Vasconcelos, M.W. Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci. 2014, 215, 134–140. [Google Scholar] [CrossRef]
- Salachna, P.; Zawadzinska, A. Effect of chitosan on plant growth, flowering and corms yield of potted. J. Ecol. Eng. 2014, 15, 97–102. [Google Scholar]
- Goñi, O.; Quille, P.; O’Connell, S. Production of chitosan oligosaccharides for inclusion in a plant biostimulant. Pure Appl. Chem. 2016, 88, 881–889. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Zhang, R.; Wang, W.; Zhao, X.; Du, Y.; Yin, H. Effects of chitosan oligosaccharides on the yield components and production quality of different wheat cultivars (Triticum aestivum L.) in Northwest China. Field Crop Res. 2015, 172, 11–20. [Google Scholar] [CrossRef]
- Kananont, N.; Pichyangkura, R.; Chanprame, S.; Chadchawan, S.; Limpanavech, P. Chitosan specificity for the in vitro seed germination of two Dendrobium orchids (Asparagales: Orchidaceae). Sci. Hortic. 2010, 124, 239–247. [Google Scholar] [CrossRef]
- Vasconcelos, M.W. Chitosan and chitooligosaccharide utilization in phytoremediation and biofortification programs: Current knowledge and future perspectives. Front. Plant Sci. 2014, 5, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nwe, N.; Furuike, T.; Tamura, H. Marine Biomaterials: Characterization, Isolation and Applications; Kim, S., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2013; Chapter 4. [Google Scholar] [CrossRef] [Green Version]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. ChemBioEng. Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczesna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Ríos, A.G.D. Technical and Economic Feasibility Analysis of a Plant Process for Production of Chitosan from Shrimp Shells in Colombia. Master’s Thesis, University of Antioquia, Medellín, Colombia, 2015. [Google Scholar]
- Gortari, M.C.; Hours, R.A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 14. [Google Scholar]
- Philibert, T.; Byong, H.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.J.; Park, R.D. Bioproduction of chitooligosaccharides: Present and perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef] [Green Version]
- Puac, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Processes Polym. 2017, 15, e1700174. [Google Scholar] [CrossRef]
- Misra, N.N.; Schluter, O.; Cullen, P.J. Cold Plasma for Food and Agriculture: Fundamentals and Applications; Elsevier: London, UK, 2016. [Google Scholar]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Air atmospheric dielectric barrier discharge plasma induced germination and growth enhancement of wheat seed. Plasma Chem. Plasma Process. 2017, 37, 1621–1634. [Google Scholar] [CrossRef]
- Gomez-Ramirez, A.; Lopez-Santos, C.; Cantos, M.; Garcia, J.L.; Molina, R.; Cotrino, J.; Espinos, J.P.; Gonzalez-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, R.; Bogaerts, A.; Bongers, W.; Fridman, A.; Fridman, G.; Locke, B.R.; Miller, V.; Reuter, S.; Schiorlin, M.; Verreycken, T.; et al. White paper on the future of plasma science in environment, for gas conversion and agriculture. Plasma Processes Polym. 2018, 16, e1700238. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Cooper, B.; Stroshine, R.L.; Ileleji, K.E.; Keener, K.M. Structures of degradation products and degradation pathways of Aflatoxin B1 by high-voltage atmospheric cold plasma (HVACP) treatment. J. Agric. Food Chem. 2017, 65, 6222–6230. [Google Scholar] [CrossRef]
- Jampala, S.N.; Manolache, S.; Gunasekaran, S.; Denes, F.Z. Plasma-enhanced modification of xanthan gum and its effect on rheological properties. J. Agric. Food Chem. 2005, 53, 3618–3625. [Google Scholar] [CrossRef]
- Dong, S.; Wang, J.; Cheng, L.; Lu, Y.; Li, S.; Chen, Y. Behavior of zein in aqueous ethanol under atmospheric pressure cold plasma treatment. J. Agric. Food Chem. 2017, 65, 7352–7360. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.; Kim, H.J.; Jo, C. Plasma-induced degradation of quercetin associated with the enhancement of biological activities. J. Agric. Food Chem. 2017, 65, 6929–6935. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Ren, L.; Wang, Y. Argon plasma-induced graft polymerization of PEGMA on chitosan membrane surface for cell adhesion improvement. Plasma Sci. Technol. 2013, 15, 1041–1046. [Google Scholar] [CrossRef] [Green Version]
- Pankaj, S. Cold Plasma Treatment of Biodegradable Films and Smart Packaging. Ph.D. Thesis, Dublin Institute of Technology, Dublin, Ireland, 2015. [Google Scholar]
- Vosmanská, V.; Kolářová, K.; Rimpelová, S.; Kolská, Z.; Švorčík, V. Antibacterial wound dressing: Plasma treatment effect on chitosan impregnation and in situ synthesis of silver chloride on cellulose surface. RSC Adv. 2015, 5, 17690–17699. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, D.; Lipatova, I.; Naumova, I.; Sirotkin, N.; Pleskunov, P.; Krakovský, I.; Khalakhan, I.; Choukourov, A.; Titov, V.; Agafonov, A. Immobilization of chitosan onto polypropylene foil via air/solution atmospheric pressure plasma afterglow treatment. Plasma Chem. Plasma Process. 2019, 40, 207–220. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Rujiravanit, R.; Watthanaphanit, A.; Theeramunkong, S.; Saito, N.; Yamashita, K.; Arakawa, R. Enhanced degradation of chitosan by applying plasma treatment in combination with oxidizing agents for potential use as an anticancer agent. Carbohydr. Polym. 2017, 167, 1–11. [Google Scholar] [CrossRef]
- Prasertsung, I.; Damrongsakkul, S.; Saito, N. Degradation of β-chitosan by solution plasma process (SPP). Polym. Degrad. Stab. 2013, 98, 2089–2093. [Google Scholar] [CrossRef]
- Nikitin, D.; Choukourov, A.; Titov, V.; Kuzmicheva, L.; Lipatova, L.; Mezina, E.; Aleksandriiskii, V.; Shelemin, A.; Khalakhan, I.; Slavinska, D.; et al. In situ coupling of chitosan onto polypropylene foils by an Atmospheric Pressure Air Glow Discharge with a liquid cathode. Carbohydr. Polym. 2016, 154, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Vasilieva, T.; Sigarev, A.; Kosyakov, D.; Ul’yanovskii, N.; Anikeenko, E.; Chuhchin, D.; Ladesov, A.; Hein, A.M.; Miasnikov, V. Formation of low molecular weight oligomers from chitin and chitosan stimulated by plasma-assisted processes. Carbohydr. Polym. 2017, 163, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, M.; Vasilieva, T.; Hein, A.M. Hybrid plasma chemical reactors for bio-polymers processing. J. Phys. D Appl. Phys. 2019, 52, 335202. [Google Scholar] [CrossRef]
- Vasiliev, M.; Vasilieva, T. Encyclopedia of Plasma Technology; Shohet, J.L., Ed.; CRC Press, Taylor and Francis Inc.: Boka Ralton, FL, USA, 2016; pp. 152–166. [Google Scholar]
- Aleksandrov, N.L.; Vasil’ev, M.N.; Lysenko, S.L.; Makhir, A.K. Experimental and theoretical study of a quasi-steady electron-beam plasma in hot argon. Plasma Phys. Rep. 2005, 31, 425–435. [Google Scholar] [CrossRef]
- Vasilieva, T.M.; Vasiliev, M.N.; Garaeva, V.V.; Zlobin, I.S.; Mint, Z.Y.; Htau, K.M.; Kyaw, H.W.Y.; Zaw, H.K.K. Hybrid plasma—Prospects for applications in medicine and biology. Russ. Phys. J. 2020, 62, 2092–2100. [Google Scholar] [CrossRef]
- Vasilieva, T.; Chuhchin, D.; Lopatin, S.; Varlamov, V.; Sigarev, A.; Vasiliev, M. Chitin and cellulose processing in low-temperature electron beam plasma. Molecules 2017, 22, 1908. [Google Scholar] [CrossRef] [Green Version]
- Quackenbos, H.M. Relation between intrinsic viscosity and molecular weight. J. Appl. Polym. Sci. 1980, 25, 1435–1442. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharmaceut. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Burrell, T.; Fozard, S.; Holroyd, G.H.; French, A.P.; Pound, M.P.; Bigley, C.J.; Forde, B.G. The Microphenotron: A robotic miniaturized plant phenotyping platform with diverse applications in chemical biology. Plant Methods 2017, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Carmody, N.; Goñi, O.; Łangowski, Ł.; O’Connell, S. Ascophyllum nodosum extract biostimulant processing and its impact on enhancing heat stress tolerance during tomato fruit set. Front. Plant Sci. 2020, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant Sci. 2018, 9, 428. [Google Scholar] [CrossRef]
- Yin, H.; Du, Y.; Dong, Z. Chitin oligosaccharide and chitosan oligosaccharide: Two similar but different plant elicitors. Front. Plant Sci. 2016, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Qandil, A.M.; Marjic, T.J.; Al-Taanid, B.M.; Khalede, A.H.; Badwan, A.A. Depolymerization of HMW into a predicted LMW chitosan and determination of the degree of deacetylation to guarantee its quality for research use. J. Excip. Food Chem. 2018, 9, 51–63. [Google Scholar]
- Kim, S.K.; Je, J.Y. Continuous Production of Chitooligosaccharides by Enzymatic Hydrolysis in Chitin, Chitosan and Their Derivatives: Biological Activities and Applications; Kim, S., Ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2010; p. 47. [Google Scholar]
- Lopez-Moya, F.; Escudero, N.; Zavala-Gonzalez, E.A.; Esteve-Bruna, D.; Blázquez, M.A.; Alabadí, D.; Lopez-Llorca, L.V. Induction of auxin biosynthesis and WOX5 repression mediate changes in root development in Arabidopsis exposed to chitosan. Sci. Rep. 2017, 7, 16813. [Google Scholar] [CrossRef] [Green Version]
- Asghari-Zakaria, R.; Maleki-Zanjani, B.; Sedghi, E. Effect of in vitro chitosan application on growth and minituber yield of Solanum tuberosum L. Plant Soil Environ. 2009, 55, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Dzung, P.D.; Phu, D.V.; Du, B.D.; Ngoc, L.S.; Duy, N.N.; Hiet, H.D.; Nghia, D.H.; Thang, N.T.; Le, B.V.; Hien, N.Q. Effect of foliar application of oligochitosan with different molecular weight on growth promotion and fruit yield enhancement of chili plant. Plant Prod. Sci. 2017, 20, 389–395. [Google Scholar] [CrossRef]
- Sultana, S.; Islam, M.; Khatun, M.A.; Hassain, M.A.; Huque, R. Effect of foliar application of oligo-chitosan on growth, yield and quality of tomato and eggplant. Asian J. Agric. Res. 2017, 11, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Uddin, I.; Islam, J.M.M.; Haque, A.; Zubair, A.; Barua, R.; Rahaman, S.; Rahman, L.; Khan, M.A. Significant influence of gamma-radiation-treated chitosan and alginate on increased productivity as well as improved taste and flavor of pineapple. Int. J. Fruit Sci. 2020, 20, 455–469. [Google Scholar] [CrossRef]
- Moller, M.N.; Li, Q.; Vitturi, D.A.; Robinson, J.M.; Lancaster, J.R.; Denicola, A. Membrane “lens” effect: Focusing the formation of reactive nitrogen oxides from the *NO/O2 reaction. Chem. Res. Toxicol. 2007, 20, 709–714. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Kasper, D.L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 2011, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Boric, M.; Puliyalil, H.; Novak, U.; Likozar, B. An intensified atmospheric plasma-based process for the isolation of the chitin biopolymer from waste crustacean biomass. Green Chem. 2018, 20, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Xin, R.; Xie, W.; Xu, Z.; Che, H.; Zheng, Z.; Yang, X. Efficient extraction of chitin from shrimp waste by mutagenized strain fermentation using atmospheric and room-temperature plasma. Int. J. Biol. Macromol. 2019. [Google Scholar] [CrossRef]
- Tantiplapol, T.; Singsawat, Y.; Narongsil, N.; Damrongsakkul, S.; Saito, N.; Prasertsung, I. Influences of solution plasma conditions on degradation rate and properties of chitosan. Innov. Food Sci. Emerg. Technol. 2015, 32, 116–120. [Google Scholar] [CrossRef]
- Titov, V.A.; Lipatova, I.M.; Mezina, E.A.; Kuz’micheva, L.A. Plasma-chemical destruction and modification of chitosan in solution. High Energy Chem. 2016, 50, 411–415. [Google Scholar] [CrossRef]
- Na, L.; Yingchun, F.; Jie, L.; Yan, S.; Kefeng, S.; Yan, W. Electrical characteristics of pulsed corona discharge plasmas in chitosan solution. Plasma Sci. Technol. 2014, 16, 128–133. [Google Scholar] [CrossRef]
- Trombotto, S.; Ladavière, C.; Delolme, F.; Domard, A. Chemical preparation and structural characterization of a homogeneous series of chitin/chitosan oligomers. Biomacromolecules 2008, 9, 1731–1738. [Google Scholar] [CrossRef]
- Grifoll-Romero, L.; Pascual, S.; Aragunde, H.; Biarnés, X.; Planas, A. Chitin deacetylases: Structures, specificities, and biotech applications. Polymers 2018, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaeni, A.; Safitri, E.; Fuadah, B.; Sudiana, I.N. Microwave-assisted hydrolysis of chitosan from shrimp shell waste for glucosammine hydrochlorid production. J. Phys. Conf. Ser. 2017, 846, 012011. [Google Scholar] [CrossRef] [Green Version]
- Piątkowski, M.; Janus, Ł.; Radwan-Pragłowska, J.; Raclavsky, K. Microwave-enhanced synthesis of biodegradable multifunctional chitosan hydrogels for wastewater treatment. eXPRESS Polym. Lett. 2017, 11, 809–819. [Google Scholar] [CrossRef]
- National Research Council. Microwave Processing of Materials; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar] [CrossRef] [Green Version]
- Kalganova, S.G.; Arkhangelsky, Y.S.; Lavrentiev, V.A.; Trigorly, S.V. Scientific basis for modification of polymer materials in a microwave electromagnetic field. J. Electrotech. 2017, 14, 26–35. [Google Scholar]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Microwave pyrolysis of polymeric materials. In Microwave Heating; Chandra, U., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 207–232. [Google Scholar]
- Vasiliev, M.N.; Mahir, A.H. Electron-beam plasma systems in industrial and aerospace applications. In Proceedings of the 24th Summer School and International Symposium on the Physics of Ionized Gases, Novi Sad, Serbia, 25–29 August 2008; pp. 421–426. [Google Scholar]
- Vasilieva, T.M.; Bayandina, D.V. An experimental complex for studying the operation of Beam-Plasma Reactors for biomedical applications. Instrum. Exp. Tech. 2010, 53, 289–296. [Google Scholar] [CrossRef]






| Parameter | Value or Characteristics |
|---|---|
| Accelerating voltage (Eb) | 30 kV |
| EB current (Ib) | 1.5–2.0 mA |
| Oxygen pressure (Pm) | 0.2–1.0 kPa |
| The EB scanning mode | Concentric ellipses |
| The reactor loading | ≈10 g per cycle |
| The distance between the injection window and front end of the reaction chamber | 150–250 mm depending on gas pressure, Pm |
| Treatment time (τ) | 5–10 min |
| Material temperature (Ts) | ≈40 °C |
| Plasma density (nep) | 108–1010 cm–3 |
| Method of the COS Production | ||||
|---|---|---|---|---|
| Characteristic | EBP-Processing | Chemical Hydrolysis | Enzyme Hydrolysis | |
| 5 min | 10 min | |||
| Molecular weight Mw, Da | 1300 | 570 | Below 3900 [14,15] | The COS with degrees of polymerization between 2 and 20 (500–5000 Da) are produced, depending on the hydrolysis conditions and enzyme used [14,15] |
| Polydispersity | 1.6 | 1.5 | 1.0–2.5 [52] | 1.0–2.6 [52] |
| DD, % | 94.8 ± 2.6 | 96.0 ± 2.7 | 96–98 depending on the hydrolysis conditions and original chitosan characteristics [46] | 58–99 depending on the hydrolysis conditions and enzyme used [53] |
| Water-solubility, % | 95 | 95 | 90–100 [14,15] | 90–100 [14,15] |
| Criteria | Chemical Hydrolysis | Enzymatic Hydrolysis | EBP-Processing |
|---|---|---|---|
| Treatment duration | Several hours or days | Several hours | Minutes (τ = 2–10) |
| Number of stages | Multi-stage | Multi-stage | Single-stage |
| Specificity and efficiency |
|
|
|
| COS yields | Low yields of COS and large amounts of monomeric units [53]. | Up to 90–95% [14,15,51]. | Up to 80–85%. |
| Environment safety |
| Environmentally friendly. However, diluted acidic and alkaline solution may be needed [14,34]. | Environmentally friendly. Hazardous by-products and toxic wastes are not generated. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilieva, T.; Goñi, O.; Quille, P.; O’Connell, S.; Kosyakov, D.; Shestakov, S.; Ul’yanovskii, N.; Vasiliev, M. Chitosan Plasma Chemical Processing in Beam-Plasma Reactors as a Way of Environmentally Friendly Phytostimulants Production. Processes 2021, 9, 103. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9010103
Vasilieva T, Goñi O, Quille P, O’Connell S, Kosyakov D, Shestakov S, Ul’yanovskii N, Vasiliev M. Chitosan Plasma Chemical Processing in Beam-Plasma Reactors as a Way of Environmentally Friendly Phytostimulants Production. Processes. 2021; 9(1):103. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9010103
Chicago/Turabian StyleVasilieva, Tatiana, Oscar Goñi, Patrick Quille, Shane O’Connell, Dmitry Kosyakov, Semen Shestakov, Nikolay Ul’yanovskii, and Michael Vasiliev. 2021. "Chitosan Plasma Chemical Processing in Beam-Plasma Reactors as a Way of Environmentally Friendly Phytostimulants Production" Processes 9, no. 1: 103. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9010103