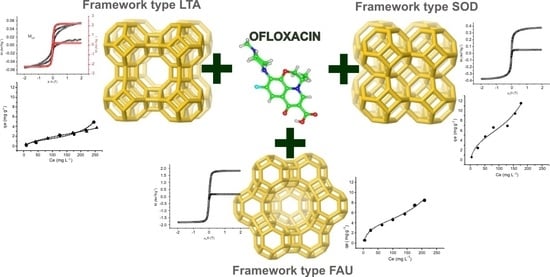
Efficiency in Ofloxacin Antibiotic Water Remediation by Magnetic Zeolites Formed Combining Pure Sources and Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zeolite Synthesis
2.3. Characterization of Wastes and Zeolites
2.4. Analytical Measurements
2.5. Adsorption and Kinetic Experiments
3. Results
3.1. Magnetic Properties of Synthetic Zeolites
3.2. Adsorption and Kinetic Behavior
3.2.1. Preliminary Experiments
3.2.2. Adsorption Experiments
3.2.3. Ofloxacin Adsorption under Environmental Conditions
4. Conclusions
Supplementary Materials
 ), SARMs (
), SARMs (  ), FAnM (
), FAnM (  ), and FARMp (
), and FARMp (  ) (Experimental conditions: 10 mL tap water, OFL concentration 10 L−1), Figure S5: Adsorption profiles Langmuir (--), Freundlich (_) and BET (…) for OFL on (a) FARMp, (b) FanM, (c) SARMs and (d) SAnM, (Experimental conditions: 100 mg, 10 mL tap water, OFL solution from 10 to 293 mg L−1), Figure S6: Kinetic profiles (pseudo-first order (_) and pseudo-second (..) order) for OFL on (a) FARMp, (b) FAnM, (c) SARMs and (d) SAnM (Experimental conditions: 200 mg, 20 mL tap water, OFL initial concentration 20 mg L−1), Table S1: Sample synthesis, Table S2: XRF chemical composition of red muds (RM) and fly ash (FA) (wt%), Table S3: Isotherm parameters obtained by fitting the experimental data for OFL adsorption onto SAnM, SARMs, FAnM, and FARMp. (Experimental conditions: 100 mg adsorbent phase, 10 mL tap water, OFL concentrations from 10 to 293 mg L−1), Table S4: Municipal water of Pavia and Ticino River physico-chemical parameters. Conductivity (µS cm−1); other parameters (mg L−1).
) (Experimental conditions: 10 mL tap water, OFL concentration 10 L−1), Figure S5: Adsorption profiles Langmuir (--), Freundlich (_) and BET (…) for OFL on (a) FARMp, (b) FanM, (c) SARMs and (d) SAnM, (Experimental conditions: 100 mg, 10 mL tap water, OFL solution from 10 to 293 mg L−1), Figure S6: Kinetic profiles (pseudo-first order (_) and pseudo-second (..) order) for OFL on (a) FARMp, (b) FAnM, (c) SARMs and (d) SAnM (Experimental conditions: 200 mg, 20 mL tap water, OFL initial concentration 20 mg L−1), Table S1: Sample synthesis, Table S2: XRF chemical composition of red muds (RM) and fly ash (FA) (wt%), Table S3: Isotherm parameters obtained by fitting the experimental data for OFL adsorption onto SAnM, SARMs, FAnM, and FARMp. (Experimental conditions: 100 mg adsorbent phase, 10 mL tap water, OFL concentrations from 10 to 293 mg L−1), Table S4: Municipal water of Pavia and Ticino River physico-chemical parameters. Conductivity (µS cm−1); other parameters (mg L−1).Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the subcommittee on zeolites of the International Mineralogical Association, Commission on New Minerals and Mineral Names. Can. Mineral. 1997, 35, 1571–1606. [Google Scholar]
- Čejka, J.; Millini, R.; Opanasenko, M.; Serrano, D.P.; Roth, J. Advances and challenges in zeolite synthesis and catalysis. Catal. Today 2020, 345, 2–13. [Google Scholar] [CrossRef]
- Bellussi, G.; Carati, A.; Rizzo, C.; Millini, R. New trends in the synthesis of crystalline microporous materials. Catal. Sci. Tech. 2013, 3, 833–857. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Moliner, M.; Corma, A. Building zeolites from precrystallized units: Nanoscale architecture. Angew. Chem. Int. Ed. 2018, 57, 15330–15353. [Google Scholar] [CrossRef]
- Oste, L.A.; Lexmond, T.M.; Van Riemsdijk, W.H. Metal immobilization in soils using synthetic zeolites. J. Environ. Qual. 2002, 31, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Hajabbasi, M.A.; Khademi, H.; Kazemian, H. Soil cadmium stabilization using an Iranian natural zeolite. Geoderma 2007, 137, 388–393. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Shao, H.; Shao, M. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Ni by synthesising zeolite at low temperatures in a polluted soil. Chemosphere 2010, 78, 1172–1176. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Micropor. Mesopor. Mat. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Ragone, P.; Fiore, S. Immobilization of Zn and Pb in polluted soil by in-situ crystallization zeolites from fly ash. Water Air Soil Pollut. 2012, 223, 5357–5364. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Use of zeolite to neutralise nickel in a soil environment. Environ. Monit. Assess. 2018, 190, 54. [Google Scholar] [CrossRef] [Green Version]
- Belviso, C. Zeolite for potential toxic metals uptake from contaminated soil: A brief review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namiesnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Maretto, M.; Blanchi, F.; Vignola, R.; Canepari, S.; Baric, M.; Iazzoni, R.; Tagliabue, M.; Papini, M.P. Microporous and mesoporous materials for the treatment of wastewater produced by petrochemical activities. J. Clean. Prod. 2014, 77, 22–34. [Google Scholar] [CrossRef]
- Martucci, A.; Braschi, I.; Marchese, L.; Quartieri, S. Recent advances in clean-up strategies of waters polluted with sulfonamide antibiotics: A review of sorbents and related properties. Mineral. Mag. 2014, 78, 1115–1140. [Google Scholar] [CrossRef]
- Bandura, L.; Woszuk, A.; Kołodynska, D.; Franus, W. Application of mineral sorbents for removal of petroleum substances: A review. Minerals 2017, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Mancinelli, M.; Arfè, A.; Martucci, A.; Pasti, L.; Chenet, T.; Sarti, E.; Vergine, G.; Belviso, C. Evaluation for the removal efficiency of VOCs and heavy metals by zeolites-based materials in the wastewater: A case study in the Tito Scalo industrial area. Processes 2020, 8, 1519. [Google Scholar] [CrossRef]
- Rodeghero, E.; Chenet, T.; Martucci, A.; Ardit, M.; Sarti, E.; Pasti, L. Selective adsorption of toluene and n-hexane binary mixture from aqueous solution on zeolite ZSM-5: Evaluation of competitive behavior between aliphatic and aromatic compounds. Catal. Today 2020, 345, 157–164. [Google Scholar] [CrossRef]
- Mintova, S.; Olson, N.H.; Valtchev, V.; Bein, T. Nanocrystal growth from colloids at room temperature. Science 1999, 28, 958–960. [Google Scholar] [CrossRef]
- Matthew, D.O.; Soltis, J.A.; Marlon, T.C.; Lee Penn, R.; Rimer, J.D. Nucleation of FAU and LTA zeolites from heterogeneous aluminosilicate precursors. Chem. Mater. 2016, 28, 4906–4916. [Google Scholar]
- Belviso, C. EMT-type zeolite synthesized from obsidian. Micropor. Mesopor. Mat. 2016, 226, 325–330. [Google Scholar] [CrossRef]
- Belviso, C.; Peddis, D.; Varvaro, G.; Abdolrahimi, M.; Reverberi, A.P.; Cavalcante, F. Obsidian as raw material for eco-friendly synthesis of magnetic zeolite. Materials 2020, 13, 4633. [Google Scholar] [CrossRef]
- Psycharis, V.; Perdikatsis, V.; Christidis, G. Crystal structure and Rietveld refinement of zeolite A synthesized from fine-grained perlite waste materials. Bull. Geol. Soc. Greece 2004, 36, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Shawabkeh, R.; Al-Harahsheh, A.; Hami, M.; Khlaifat, A. Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater. Fuel 2004, 83, 981–985. [Google Scholar] [CrossRef]
- Qian, T.; Li, J. Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Tan, K.H.; Adam, F.; Retoux, R.; Mintova, S. EMT-type zeolite nanocrystals synthesized from rice husk. Micropor. Mesopor. Mat. 2015, 204, 2014–2209. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Z.; Guo, M.; Zhang, M.; Liu, J. Feasible conversion of solid waste bauxite tailings into highly crystalline 4A zeolite with valuable application. Waste Manag. 2014, 34, 2365–2372. [Google Scholar] [CrossRef]
- Liu, L.; Du, T.; Li, G.; Yang, F.; Che, S. Using one waste to tackle another: Preparation of a CO2 capture material zeolite X from laterite residue and bauxite. J. Hazard. Mater. 2014, 278, 551–558. [Google Scholar] [CrossRef] [Green Version]
- Belviso, C.; Agostinelli, E.; Belviso, S.; Cavalcante, F.; Pascucci, S.; Peddis, D.; Varvaro, G.; Fiore, S. Synthesis of magnetic zeolite at low temperature using a waste material mixture: Fly ash and red mud. Micropor. Mesopor. Mat. 2015, 202, 208–216. [Google Scholar] [CrossRef]
- Belviso, C.; Kharchenko, A.; Agostinelli, E.; Cavalcante, F.; Peddis, D.; Varvaro, G.; Yaacoub, N.; Mintova, S. Red mud as aluminium source for the synthesis of magnetic zeolite. Micropor. Mesopor. Mat. 2018, 270, 24–29. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, L.; Jiang, Z.; Liu, C.; Zhang, Q.; Zou, Y.; Chen, Y.; Li, J.; Liu, X. Feasible low-cost conversion of red mud into magnetically separated and recycled hybrid SrFe12O19@ NaP1 zeolite as a novel wastewater adsorbent. Chem. Eng. J. 2020, 417, 128090. [Google Scholar] [CrossRef]
- Belviso, C.; Cannas, C.; Pinna, N.; Cavalcante, F.; Lettino, A.; Lotti, P.; Gatta, G.D. Effect of red mud added to zeolite LTA synthesis: Where is Fe in the newly-formed material? Micropor. Mesopor. Mat. 2020, 298, 1100583. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Petkowicz, D.I.; Smaniotto, A.; Pergher, S.B.C. Magnetic zeolites: A new adsorbent for removal of metallic contaminants from water. Water Res. 2004, 38, 3699–3704. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 2015, 270, 393–404. [Google Scholar] [CrossRef]
- Alberti, S.; Caratto, V.; Peddis, D.; Belviso, C.; Ferretti, M. Synthesis and characterization of a new photocatalyst based on TiO2 nanoparticles supported on a magnetic zeolite from iron and steel industrial waste. J. Alloys Compd. 2019, 797, 820–825. [Google Scholar] [CrossRef]
- Faghihian, H.; Moayed, M.; Firooz, A.; Iravani, M. Synthesis of a novel magnetic zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Colloid Interface Sci. 2013, 393, 445–451. [Google Scholar] [CrossRef]
- Ifthikar, J.; Wang, J.; Wang, Q.; Wang, T.; Wang, H.; Khan, A.; Jawad, A.; Sun, T.; Jiao, X.; Chen, Z. Highly efficient lead distribution by magnetic sewage sludge biochar: Sorption mechanisms and bench applications. Biores. Technol. 2017, 238, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Braschi, I.; Blasioli, S.; Gigli, L.; Gessa, C.E.; Alberti, A.; Martucci, A. Removal of sulfonamide antibiotics from water: Evidence of adsorption into an organophilic zeolite Y by its structural modifications. J. Hazard. Mat. 2010, 178, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Martucci, A.; Pasti, L.; Marchetti, N.; Cavazzini, A.; Dondi, F.; Alberti, A. Adsorption of pharmaceuticals from aqueous solutions on synthetic zeolites. Micropor. Mesopor. Mat. 2012, 148, 174–183. [Google Scholar] [CrossRef]
- Deo, R.P. Pharmaceuticals in the surface water of the USA: A review. Curr. Environ. Health Rep. 2014, 1, 113–122. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Yazdanbakhsh, A.R.; Rastkari, N.; Mohseni-Bandpei, A.; Nasseri, S.; Piroti, E.; Asadi, A. Occurrence of non-steroidal anti-inflammatory drugs in Tehran source water, municipal and hospital wastewaters, and their ecotoxicological risk assessment. Environ. Monit. Assess. 2015, 187, 1–15. [Google Scholar] [CrossRef]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Mass balance of emerging contaminants in the water cycle of a highly urbanized and industrialized area of Italy. Water Res. 2018, 131, 287–298. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara, G.G.; Chaminda, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Ground W. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Fernando, M.; Pereira, R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- European Commission, Communication from the Commission to the European Parliament, the Council, and the European Economic and Social Committee: European Union Strategic Approach to Pharmaceuticals in the Environment. Communication 2019, 128, 13.
- Merlin, C. Reducing the consumption of antibiotics: Would that be enough to slow down the dissemination of resistances in the downstream environment? Front. Microbiol. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturini, M.; Maraschi, F.; Cantalupi, A.; Pretali, L.; Nicolis, S.; Dondi, D.; Profumo, A.; Caratto, V.; Sanguineti, E.; Ferretti, M.; et al. TiO2 and N-TiO2 sepiolite and zeolite composites for photocatalytic removal of ofloxacin from polluted water. Materials 2020, 13, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Sarti, E.; Chenet, T.; Stevanin, C.; Costa, V.; Cavazzini, A.; Catani, M.; Martucci, A.; Precisvalle, N.; Beltrami, G.; Pasti, L. High-silica zeolites as sorbent media for adsorption and pre-concentration of pharmaceuticals in aqueous solutions. Molecules 2020, 25, 3331. [Google Scholar] [CrossRef]
- Rivagli, E.; Pastorello, A.; Sturini, M.; Maraschi, F.; Speltini, A.; Zampori, L.; Setti, M.; Malavasi, L.; Profumo, A. Clay minerals for adsorption of veterinary FQs: Behavior and modelling. J. Environ. Chem. Eng. 2014, 2, 738–744. [Google Scholar] [CrossRef]
- Maraschi, F.; Sturini, M.; Speltini, A.; Pretali, L.; Profumo, A.; Pastorello, A.; Kumar, V.; Ferretti, M.; Caratto, V. TiO2-modified zeolites for fluoroquinolones removal from wastewaters and re-use after solar light regeneration. J. Environ. Chem. Eng. 2014, 2, 2170–2176. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Tarantino, S.; Gualtieri, A.F.; Zema, M. Removal of fluoroquinolone contaminants from environmental waters on sepiolite and its photo-induced regeneration. Chemosphere 2016, 150, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, D.; Guerra, G.; Puscalau, C.; Maraschi, F.; Bruni, G.; Monteforte, F.; Profumo, A.; Sturini, M. Zinc based metal-organic frameworks as ofloxacin adsorbents in polluted waters: ZIF-8 vs. Zn3(BTC)2. Int. J. Environ. Res. Public Health 2021, 18, 1433. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Helbling, D.E.; Dichtel, W.R. Best practices for evaluating new materials as adsorbents for water treatment. ACS Mater. Lett. 2020, 2, 1532–1544. [Google Scholar] [CrossRef]
- Liu, Q.; Barrón, V.; Torrent, J.; Qin, H.; Yu, Y. The magnetism of micro-sized hematite explained. Phys. Earth Planet. Inter. 2010, 183, 387–397. [Google Scholar] [CrossRef]
- Robberson, K.A.; Waghe, A.B.; Sabatini, D.A.; Butler, E.C. Adsorption of the quinolone antibiotic nalidixic acid onto anion-exchange and neutral polymers. Chemosphere 2006, 63, 934–941. [Google Scholar] [CrossRef]
- Mahapatra, S.; Venugopala, K.N.; Guru, T.N.; Row, A. A device to crystallize organic solids: Structure of ciprofloxacin, midazolam, and ofloxacin as targets. Cryst. Growth Des. 2010, 10, 1866–1870. [Google Scholar] [CrossRef]
- Gramlich, V.; Meier, W.M. The crystal structure of hydrated NaA: A detailed refinement of a pseudosymmetric zeolite structure. Z. Kristallogr. 1971, 133, 134–149. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Fiore, S. Synthesis of zeolite from Italian coal fly ash. Differences in crystallization temperature using seawater instead of distilled water. Waste Manag. 2010, 30, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Bruker AXS. DIFFRAC. EVA V5.1; Bruker AXS GmbH: Karlsruhe, Germany, 2019. [Google Scholar]
- ASTM. Standard Practice for Determination of Adsorption Capacity of Activated Carbon by Aqueous Phase Isotherm Technique; American Society of Testing and Materials; Annual Book of ASTM Standards; ASTM: West Conshohocken, PA, USA, 1998. [Google Scholar]
- Walker, M.; Majo, P.I.; O’Grady, K.; Charles, S.W.; Chantrell, R.W. The magnetic properties of single-domain particles with cubic anisotropy. II. Remanence curves. J. Phys. Condens. Matter. 1993, 5, 2793. [Google Scholar] [CrossRef]
- Laureti, S.; Varvaro, G.; Fiorani, D.; Agostinelli, E.; Piccaluga, G.; Musinu, A.; Ardu, A.; Peddis, D. Magnetic interactions in silica coated nanoporous assemblies of CoFe2O4 nanoparticles with cubic magnetic anisotropy. Nanotechnology 2010, 21, 315701. [Google Scholar] [CrossRef]
- Suber, P.; Imperatori, P.; Mari, A.; Marchegiani, G.; Mansilla, M.V.; Fiorani, D.; Plunkett, W.R.; Rinaldi, D.; Cannas, C.; Ennas, G.; et al. Thermal hysteresis of Morin transition in hematite particles. Phys. Chem. Chem. Phys. 2010, 12, 6984–6989. [Google Scholar] [CrossRef] [PubMed]
- Loens, J.; Schulz, H.; Strukturverfeinerung von Sodalith, H. Na8Si6Al6O24Cl2. Acta Crystallogr. 1967, 23, 434–436. [Google Scholar]
- Baur, W.H. On the cation and water positions in faujasite. Am. Mineral. 1964, 49, 697–704. [Google Scholar]
- Baerlocher, C.H.; McCusker, L.B. Database of Zeolite Structures. 2017. Available online: http://www.iza-structure.org/databases/ (accessed on 24 November 2021).





 ), SARMs (
), SARMs (  ), FAnM (
), FAnM (  ), and FARMp (
), and FARMp (  ) (Experimental conditions: adsorbent 100 mg, 10 mL tap water, OFL concentration 10–293 mg L−1).
) (Experimental conditions: adsorbent 100 mg, 10 mL tap water, OFL concentration 10–293 mg L−1).
 ), SARMs (
), SARMs (  ), FAnM (
), FAnM (  ), and FARMp (
), and FARMp (  ) (Experimental conditions: adsorbent 100 mg, 10 mL tap water, OFL concentration 10–293 mg L−1).
) (Experimental conditions: adsorbent 100 mg, 10 mL tap water, OFL concentration 10–293 mg L−1).
| Sample | Ms (Am2kg−1) | Hc (mT) | HCr (mT) |
|---|---|---|---|
| SAnM | 2.2 (5) | 11.0 (1) | 36.0 (2) |
| SARMs | 0.07 (5) | 27.0 (1) | 244.0 (5) |
| FAnM | 1.9 (5) | 10.0 (1) | 35.0 (2) |
| FARMp | 0.4 (5) | 10.0 (1) | 31.0 (2) |
| SPIONs | 88.2 (5) | 11.0 (1) | 32.0 (2) |
| R% | ||
|---|---|---|
| Zeolite | Tap Water | River Water |
| FAnM | 90 | 83 |
| FARMp | 92 | 87 |
| SAnM | 45 | 43 |
| SARMs | 63 | 61 |
| RSD % ≤ 10% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belviso, C.; Guerra, G.; Abdolrahimi, M.; Peddis, D.; Maraschi, F.; Cavalcante, F.; Ferretti, M.; Martucci, A.; Sturini, M. Efficiency in Ofloxacin Antibiotic Water Remediation by Magnetic Zeolites Formed Combining Pure Sources and Wastes. Processes 2021, 9, 2137. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122137
Belviso C, Guerra G, Abdolrahimi M, Peddis D, Maraschi F, Cavalcante F, Ferretti M, Martucci A, Sturini M. Efficiency in Ofloxacin Antibiotic Water Remediation by Magnetic Zeolites Formed Combining Pure Sources and Wastes. Processes. 2021; 9(12):2137. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122137
Chicago/Turabian StyleBelviso, Claudia, Giulia Guerra, Maryam Abdolrahimi, Davide Peddis, Federica Maraschi, Francesco Cavalcante, Maurizio Ferretti, Annalisa Martucci, and Michela Sturini. 2021. "Efficiency in Ofloxacin Antibiotic Water Remediation by Magnetic Zeolites Formed Combining Pure Sources and Wastes" Processes 9, no. 12: 2137. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9122137