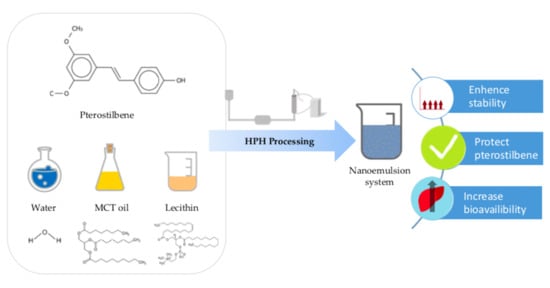
Making Concentrated Pterostilbene Highly Bioavailable in Pressure Processed Phospholipid Nanoemulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility Study
2.3. Determination of Emulsion Formulation Using the Pseudo-Ternary Phase Diagram
2.4. Preparation of Pterostilbene Nanoemulsion
2.5. Particle Size and Zeta Potential Analysis
2.6. Viscosity of Pterostilbene Nanoemulsion
2.7. HPLC Analysis of Pterostilbene
2.8. Encapsulation Efficiency of Pterostilbene Nanoemulsion
2.9. In Vitro Lipolysis of Nanoemulsion
2.10. DPPH Antioxidant Analysis
2.11. Stability Test
2.12. Cell Culture
2.13. Cell Viability Test
2.14. Caco-2 Transport Assay
2.15. Statistical Analysis
3. Results and Discussion
3.1. Solubility of Pterostilbene in Different Oils
3.2. Formulation Development from the Pseudoternary Diagram
3.3. Effect of High-Pressure Homogenization on the Physical Properties and Encapsulation Efficiency of Pterostilbene Nanoemulsion
3.4. The Stability Study
3.5. Effect of Nanoemulsion on the Antioxidant Capacity of Pterostilbene
3.6. Effect of Nanoemulsion on the In Vitro Bioaccessibility of Pterostilbene
3.7. Effect of Nanoemulsion on the Transport of Pterostilbene through the Caco-2 Monolayer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Adrian, M.; Jeandet, P.; Douillet-Breuil, A.-C.; Tesson, L.; Bessis, R. Stilbene content of mature Vitis vinifera berries in response to UV-C elicitation. J. Agric. Food Chem. 2000, 48, 6103–6105. [Google Scholar] [CrossRef] [PubMed]
- Manickam, M.; Ramanathan, M.; Jahromi, M.; Chansouria, J.P.N.; Ray, A.B. Antihyperglycemic Activity of Phenolics fromPterocarpus marsupium. J. Nat. Prod. 1997, 60, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef] [PubMed]
- McCormack, D.; McFadden, D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxidative Med. Cell. Longev. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Casadesus, G. Low-dose pterostilbene, but not resveratrol, is a potent neuromodulator in aging and Alzheimer’s disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef]
- Chiou, Y.-S.; Tsai, M.-L.; Nagabhushanam, K.; Wang, Y.-J.; Wu, C.-H.; Ho, C.-T.; Pan, M.-H. Pterostilbene is More Potent than Resveratrol in Preventing Azoxymethane (AOM)-Induced Colon Tumorigenesis via Activation of the NF-E2-Related Factor 2 (Nrf2)-Mediated Antioxidant Signaling Pathway. J. Agric. Food Chem. 2011, 59, 2725–2733. [Google Scholar] [CrossRef]
- Choo, Q.-Y.; Yeo, S.C.M.; Ho, P.C.; Tanaka, Y.; Lin, H.-S. Pterostilbene surpassed resveratrol for anti-inflammatory application: Potency consideration and pharmacokinetics perspective. J. Funct. Foods 2014, 11, 352–362. [Google Scholar] [CrossRef]
- Ting, Y.; Zhao, Q.; Xia, C.; Huang, Q. Using in Vitro and in Vivo Models to Evaluate the Oral Bioavailability of Nutraceuticals. J. Agric. Food Chem. 2015, 63, 1332–1338. [Google Scholar] [CrossRef]
- Shah, P.; Bhalodia, D.; Shelat, P. Nanoemulsion: A pharmaceutical review. Syst. Rev. Pharm. 2010, 1, 24. [Google Scholar] [CrossRef]
- Aboofazeli, R. Nanometric-Scaled Emulsions (Nanoemulsions). Iran. J. Pharm. Res. 2010, 9, 325–326. [Google Scholar]
- Humphries, J.; Pizzi, D.; Sonderegger, S.E.; Fletcher, N.L.; Houston, Z.H.; Bell, C.A.; Thurecht, K.J. Hyperbranched poly (2-oxazoline) s and poly (ethylene glycol): A structure–Activity comparison of biodistribution. Biomacromolecules 2020, 21, 3318–3331. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated liposomes form stable superlubrication vectors. Langmuir 2019, 35, 6048–6054. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, A.; Maestas-Olguin, A.; Saada, E.A.; LaBauve, A.E.; Agola, J.O.; Baty, K.E.; Doudna, J.A. Engineering of monosized lipid-coated mesoporous silica nanoparticles for CRISPR delivery. Acta Biomater. 2020, 114, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Letona, C.A.M.; Luo, K.; Jeong, K.-B.; Adra, H.J.; Park, C.-S.; Kim, Y.-R. Effect of lecithin on the spontaneous crystallization of enzymatically synthesized short-chain amylose molecules into spherical microparticles. Polymers 2019, 11, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, Y.; Li, C.C.; Pan, M.-H.; Ho, C.-T.; Huang, Q. Effect of a Labile Methyl Donor on the Transformation of 5-Demethyltangeretin and the Related Implication on Bioactivity. J. Agric. Food Chem. 2013, 61, 8090–8097. [Google Scholar] [CrossRef]
- Hoeller, S.; Sperger, A.; Valenta, C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int. J. Pharm. 2009, 370, 181–186. [Google Scholar] [CrossRef]
- Klang, V.; Valenta, C. Lecithin-based nanoemulsions. J. Drug Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Gong, Q.; Yan, Z.; Duan, M. Preparation and Characterization of a Lecithin Nanoemulsion as a Topical Delivery System. Nanoscale Res. Lett. 2009, 5, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Donsì, F.; Sessa, M.; Mediouni, H.; Mgaidi, A.; Ferrari, G. Encapsulation of bioactive compounds in nanoemulsion-based delivery systems. Procedia Food Sci. 2011, 1, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Liu, B. Identification of Oxidative Products of Pterostilbene and 3′-Hydroxypterostilbene In Vitro and Evaluation of Anti-Inflammatory and Anti-Cancer Cell Proliferative Activity. Master’s Thesis, Rutgers University-Graduate School, New Brunswick, NJ, Canada, 2014. [Google Scholar]
- Diane, J.M.M.; Burgess, J. Vitamin E nanoemulsions characterization and analysis. Int. J. Pharm. 2014, 465, 455–463. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Sharma, S.; Sahni, J.K.; Ali, J.; Baboota, S. Effect of high-pressure homogenization on formulation of TPGS loaded nanoemulsion of rutin—Pharmacodynamic and antioxidant studies. Drug Deliv. 2015, 22, 541–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, H.D.; Poejo, J.; Pinheiro, A.C.; Donsì, F.; Serra, A.T.; Duarte, C.M.; Ferrari, G.; Cerqueira, M.A.; Vicente, A.A. Evaluating the behaviour of curcumin nanoemulsions and multilayer nanoemulsions during dynamic in vitro digestion. J. Funct. Foods 2018, 48, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Hubatsch, I.; E Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, Z.; Gao, C.; Du, M.; Xu, S.; Song, H.; Liu, T. Nanoemulsion for Solubilization, Stabilization, and In Vitro Release of Pterostilbene for Oral Delivery. AAPS PharmSciTech 2014, 15, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Bali, V.; Ali, M.; Ali, J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surfaces B Biointerfaces 2010, 76, 410–420. [Google Scholar] [CrossRef]
- Shi, A.-M.; Li, D.; Wang, L.-J.; Li, B.-Z.; Adhikari, B. Preparation of starch-based nanoparticles through high-pressure homogenization and miniemulsion cross-linking: Influence of various process parameters on particle size and stability. Carbohydr. Polym. 2011, 83, 1604–1610. [Google Scholar] [CrossRef]
- Yan, B.; Park, S.H.; Balasubramaniam, V.M. Influence of high pressure homogenization with and without lecithin on particle size and physicochemical properties of whey protein-based emulsions. J. Food Process. Eng. 2017, 40, e12578. [Google Scholar] [CrossRef]
- Chebil, A.; Desbrières, J.; Nouvel, C.; Six, J.-L.; Durand, A. Ostwald ripening of nanoemulsions stopped by combined interfacial adsorptions of molecular and macromolecular nonionic stabilizers. Coll. Surfaces A Physicochem. Eng. Aspects 2013, 425, 24–30. [Google Scholar] [CrossRef]
- Benlloch, M.; Obrador, E.; Valles, S.L.; Rodriguez, M.L.; Sirerol, J.A.; Alcácer, J.; Pellicer, J.A.; Salvador, R.; Cerdá, C.; Sáez, G.T.; et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid. Redox Signal. 2016, 24, 974–990. [Google Scholar] [CrossRef] [Green Version]
- Elango, B.; Dornadula, S.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene Ameliorates Streptozotocin-Induced Diabetes through Enhancing Antioxidant Signaling Pathways Mediated by Nrf2. Chem. Res. Toxicol. 2016, 29, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, R.; Hua, X.; Zhang, L.; Lu, F.; Coursey, T.G.; Pflugfelder, S.C.; Li, D.-Q. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-oxidative Pathway. Sci. Rep. 2016, 6, 19408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, E.-X.; Lin, J.-P.; Zhang, Y.; Sheng, S.-R.; Liu, H.-X.; Zhou, Y.-L.; Xu, H. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget 2017, 8, 41988–42000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; Gil-Izquierdo, A.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bethune, S.J.; Schultheiss, N.; Henck, J.-O. Improving the Poor Aqueous Solubility of Nutraceutical Compound Pterostilbene through Cocrystal Formation. Cryst. Growth Des. 2011, 11, 2817–2823. [Google Scholar] [CrossRef]
- Peng, R.-M.; Lin, G.-R.; Ting, Y.; Hu, J.-Y. Oral delivery system enhanced the bioavailability of stilbenes: Resveratrol and pterostilbene. BioFactors 2018, 44, 5–15. [Google Scholar] [CrossRef]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- Jia, L.; Wong, H. In vitro and in vivo assessment of cellular permeability and pharmacodynamics of S-nitrosylated Captopril, a nitric oxide donor. Br. J. Pharmacol. 2001, 134, 1697–1704. [Google Scholar] [CrossRef]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef]





| HPH (Number of Cycle) | Particle Size (nm) | Zeta Potential (mV) | Viscosity (cps) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| 0 | 2507.23 ± 547.31 a | −38.52 ± 0.29 cd | 15.45 ± 0.62 a | 99.08 ± 1.23 a |
| 1 | 448.17 ± 81.51 b | −38.19 ± 0.10 b | 4.20 ± 0.16 bc | 98.19 ± 1.64 a |
| 2 | 366.07 ± 57.26 b | −37.94 ± 0.15 a | 4.30 ± 0.74 bc | 99.34 ± 0.85 a |
| 3 | 212.63 ± 13.14 b | −38.63 ± 0.20 d | 4.60 ± 0.17 bc | 95.45 ± 2.34 a |
| 4 | 272.26 ± 79.31 b | −38.61 ± 0.17 d | 4.00 ± 0.17 c | 94.11 ± 7.75 a |
| 5 | 452.30 ± 35.75 b | −38.34 ± 0.20 bc | 5.20 ± 0.17 d | 98.64 ± 0.95 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.-M.; Chou, Y.-J.; Huang, Q.; Hu, J.-Y.; Ting, Y. Making Concentrated Pterostilbene Highly Bioavailable in Pressure Processed Phospholipid Nanoemulsion. Processes 2021, 9, 294. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020294
Sun F-M, Chou Y-J, Huang Q, Hu J-Y, Ting Y. Making Concentrated Pterostilbene Highly Bioavailable in Pressure Processed Phospholipid Nanoemulsion. Processes. 2021; 9(2):294. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020294
Chicago/Turabian StyleSun, Fu-Min, Yu-Jou Chou, Qingrong Huang, Jing-Yu Hu, and Yuwen Ting. 2021. "Making Concentrated Pterostilbene Highly Bioavailable in Pressure Processed Phospholipid Nanoemulsion" Processes 9, no. 2: 294. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020294