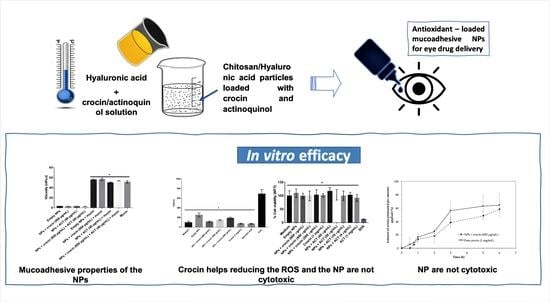
Antioxidant-Loaded Mucoadhesive Nanoparticles for Eye Drug Delivery: A New Strategy to Reduce Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Manufacturing Process
2.2.2. Nanoparticles Characterization
Encapsulation Efficiency and Drug Loading Determination
Fourier-Transform Infrared Spectroscopy (FTIR)
Transmission Electron Microscopy (TEM)
2.2.3. Stability Testing
2.2.4. In Vitro Assays
Cytotoxicity
Oxidative Stress Tests
2.2.5. In Vitro Release and Permeation Studies
- (i)
- Zero order kinetics
- (ii)
- First order kinetics
- (iii)
- Higuchi model
- (iv)
- Korsmeyer-Peppas model
2.2.6. Mucoadhesion Studies
Ostwald Viscometer
Rotational Rheometer
Zeta Potential
2.2.7. Statistical Data Analysis
3. Results and Discussion
3.1. Manufacturing Process and Nanoparticles Characterization
3.1.1. Encapsulation Efficiency and Drug Loading Determination
3.1.2. FTIR
3.1.3. TEM
3.2. Stability Tests
3.3. In Vitro Assays
3.3.1. Cytotoxicity
3.3.2. Oxidative Stress Tests
3.4. In Vitro Release and Permeation Studies
3.5. Mucoadhesive Studies
3.5.1. Ostwald Viscometer
3.5.2. Rotational Rheometer
3.5.3. Zeta Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017. [Google Scholar] [CrossRef]
- Umapathy, A.; Donaldson, P.; Lim, J. Antioxidant Delivery Pathways in the Anterior Eye. BioMed Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saccà, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental Light and Endogenous Antioxidants as the Main Determinants of Non-cancer Ocular Diseases. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Tezel, G. The Role of Glia, Mitochondria, and the Immune System in Glaucoma. Investig. Opthalmol. Vis. Sci. 2009, 50, 1001–1012. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.C.; Chiang, B.; Wu, X.; Prausnitz, M.R. Ocular Delivery of Macromolecules. J. Control. Release 2014, 190, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Malviya, R.; Sharma, P.K. Recent Trends in Ocular Drug Delivery: A Short Review. Eur. J. Appl. Sci. 2011, 3, 86–92. [Google Scholar]
- Ludwing, A. The Use of Mucoadhesive Polymers in Ocular Drug Delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The Retina as a Window to the Brain—From Eye Research to CNS Disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Bull, N.D.; Martin, K.R. Concise Review: Toward Stem Cell-Based Therapies for Retinal Neurodegenerative Diseases. STEM CELLS 2011, 29, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.P.; Chihuailaf, R.H. Antioxidants and the Integrity of Ocular Tissues. Vet. Med. Int. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Simonian, N.; Coyle, J. Oxidative Stress in Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Moghaddasi, S.M. Chemicals and Medicine Usage. J. Med. Plants Res. 2010, 4, 427–430. [Google Scholar] [CrossRef]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.; Hamza, A.A.; Daoud, S.; Khazanehdari, K.; Al Hrout, A.; Baig, B.; Chaiboonchoe, A.E.; Adrian, T.; Zaki, N.; Salehi-Ashtiani, K. Saffron-Based Crocin Prevents Early Lesions of Liver Cancer: In vivo, in vitro and Network Analyses. Recent Pat. Anticancer Drug Discov. 2016, 11, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Laabich, A.; Vissvesvaran, G.P.; Lieu, K.L.; Murata, K.; McGinn, T.E.; Manmoto, C.C.; Sinclair, J.R.; Karliga, I.; Leung, D.W.; Fawzi, A.; et al. Protective Effect of Crocin against Blue Light– and White Light–Mediated Photoreceptor Cell Death in Bovine and Primate Retinal Primary Cell Culture. Invest. Opthalmol. Vis. Sci. 2006, 47, 3156–3163. [Google Scholar] [CrossRef]
- Del Amo, E.M.; Urtti, A. Current and Future Ophthalmic Drug Delivery systems: A Shift to the Posterior Segment. Drug Discov. Today 2008, 13, 135–143. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.-C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Jones, L.; Gu, F.X. Nanomaterials for Ocular Drug Delivery. Macromol. Biosci. 2012, 12, 608–620. [Google Scholar] [CrossRef]
- Mohanraj, V.; Chen, Y. Nanoparticles - A Review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Cacciotti, I.; Chronopoulou, L.; Palocci, C.; Amalfitano, A.; Cantiani, M.; Cordaro, M.; Lajolo, C.; Callà, C.; Boninsegna, A.; Lucchetti, D.; et al. Controlled Release of 18-β-Glycyrrhetic Acid by Nanodelivery Systems Increases Cytotoxicity on Oral Carcinoma Cell Line. Nanotechnology 2018, 29, 285101. [Google Scholar] [CrossRef]
- Kashikar, V.S. Ophthalmic Mucoadhesive Polymers—A Literature Review. Int. J. Pharm. Sci. Rev. Res. 2011, 7, 68–73. [Google Scholar]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Silva, A.C.; Loboa, J.M.S. Applications of Polymeric and Lipid Nanoparticles in Ophthalmic Pharmaceutical Formulations: Present and Future Considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceci, C.; Graziani, G.; Faraoni, I.; Cacciotti, I. Strategies to Improve Ellagic Acid Bioavailability: From Natural or Semisynthetic Derivatives to Nanotechnological Approaches Based on Innovative Carriers. Nanotechnology 2020, 31, 382001. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D. Mucoadhesive Drug Delivery Systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef]
- SaraswathiI, B.; Balaji, A.; Umashankar, M. Polymers in Mucoadhesive Drug Delivery System-Latest Updates. Int. J. Pharm. Sci. Rev. Res. 2013, 5, 423–430. [Google Scholar]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [Green Version]
- Menchicchi, B.; Fuenzalida, J.P.; Hensel, A.; Swamy, M.J.; David, L.; Rochas, C.; Goycoolea, F.M. Biophysical Analysis of the Molecular Interactions between Polysaccharides and Mucin. Biomacromolecules 2015, 16, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, J.H.; Li, Z.; Oh, H.J.; Ahn, K.Y.; Yoon, K.C. Efficacy of the Mineral Oil and Hyaluronic Acid Mixture Eye Drops in Murine Dry Eye. Korean J. Ophthalmol. 2015, 29, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Apaolaza, P.S.; Delgado, D.; Del Pozo-Rodríguez, A.; Gascón, A.R.; Solinis, M.A. A Novel Gene Therapy Vector Based on Hyaluronic Acid and Solid Lipid Nanoparticles for Ocular Diseases. Int. J. Pharm. 2014, 465, 413–426. [Google Scholar] [CrossRef]
- Kogan, G.; Šoltés, L.; Stern, R.; Gemeiner, P. Hyaluronic Acid: A Natural Biopolymer with a Broad Range of Biomedical and Industrial Applications. Biotechnol. Lett. 2006, 29, 17–25. [Google Scholar] [CrossRef] [PubMed]
- 32. Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of Hyalu-ronan-based Eye Drop Formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čejke, Č.; Luyckx, J.; Čejkova, J. Central Corneal Thickness Considered an Index of Corneal Hydration of the UVB Irra-diated Rabbit Cornea as Influenced by UVB Absorber. Physiol. Res. 2012, 61, 299–306. [Google Scholar] [CrossRef]
- Čejka, Č.; Luyckx, J.; Ardan, T.; Pláteník, J.; Širc, J.; Michálek, J.; Čejková, J. The Effect of Actinoquinol with Hyaluronic Acid in Eye Drops on the Optical Properties and Oxidative Damage of the Rabbit Cornea Irradiated with UVB Rays. Photochem. Photobiol. 2010, 86, 1294–1306. [Google Scholar] [CrossRef]
- Silva, B.; Marto, J.; Braz, B.S.; Delgado, E.; Almeida, A.J.; Gonçalves, L. New nanoparticles for topical ocular delivery of erythropoietin. Int. J. Pharm. 2020, 576, 119020. [Google Scholar] [CrossRef]
- Marto, J.; Gouveia, L.; Gonçalves, L.; Gaspar, D.; Pinto, P.; Carvalho, F.; Oliveira, E.; Ribeiro, H.; Almeida, A. A Quality by Design (QbD) Approach on Starch-based Nanocapsules: A Promising Platform for Topical Drug Delivery. Colloids Surf. B: Biointerfaces 2016, 143, 177–185. [Google Scholar] [CrossRef]
- Marto, J.; Neves, Â.; Gonçalves, L.; Pinto, P.; Almeida, C.; Simões, S. Rice Water: A Traditional Ingredient with Anti-Aging Efficacy. Cosmetics 2018, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-in Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Marto, J.; Ruivo, E.; Lucas, S.; Gonçalves, L.; Simões, S.; Gouveia, L.; Felix, R.; Moreira, R.; Ribeiro, H.; Almeida, A. Starch Nanocapsules Containing a Novel Neutrophil Elastase Inhibitor with Improved Pharmaceutical Performance. Eur. J. Pharm. Biopharm. 2018, 127, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; El-Feky, G.S.; Kamel, R.; Awad, G.E. Chitosan/Sulfobutylether-β-cyclodextrin Nanoparticles as a Potential Approach for Ocular Drug Delivery. Int. J. Pharm. 2011, 413, 229–236. [Google Scholar] [CrossRef]
- Yang, L.; Gao, S.; Asghar, S.; Liu, G.; Song, J.; Wang, X.; Ping, Q.; Zhang, C.; Xiao, Y. Hyaluronic acid/chitosan Nanopar-ticles for Delivery of Curcuminoid and its in vitro Evaluation in Glioma Cells. Int. J. Biol. Macromol. 2015, 72, 1391–1401. [Google Scholar] [CrossRef]
- Organic Chemistry. IR Spectroscopy Tutorial: Aromatics. Available online: https://orgchemboulder.com/Spectroscopy/irtutor/aromaticsir.shtml (accessed on 14 September 2019).
- Anisha, B.; Biswas, R.; Chennazhi, K.; Jayakumar, R. Chitosan–hyaluronic acid/nano Silver Composite Sponges for Drug Resistant Bacteria Infected Diabetic Wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Correia, C.; Moreira-Teixeira, L.; Moroni, L.; Reis, R.; van Blitterswijk, C.; Karperien, M.; Mano, J. Chitosan Scaffolds Containing Hyaluronic Acid for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2011, 17, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Tarantilis, P.A.; Beljebbar, A.; Manfait, M.; Polissiou, M. FT-IR, FT-Raman Spectroscopic Study of Carotenoids from Saffron (Crocus sativus L.) and Some Derivatives. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 1998, 54, 651–657. [Google Scholar] [CrossRef]
- Rahaiee, S.; Shojaosadati, S.; Hashemi, M.; Moini, S.; Razavi, S. Improvement of Crocin Stability by Biodegradeble Na-noparticles of Chitosan-alginate. Int. J. Biol. Macromol. 2015, 79, 423–432. [Google Scholar] [CrossRef]
- Transmission Electron Microscopy—An Overview. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/topics/food-science/transmission-electron-microscopy (accessed on 31 July 2019).
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C 2019, 123, 16495–16507. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Lopes, R.M.; Clemente, P.; Raposo, S.; Gonçalves, L.M.D.; Bica, A.; Ribeiro, H.M.; Almeida, A.J. Lecithin and Parabens Play a Crucial Role in Tripalmitin-based Lipid Nanoparticle Stabilization Throughout Moist Heat Sterilization and Freeze-drying. Eur. J. Lipid Sci. Technol. 2015, 117, 1947–1959. [Google Scholar] [CrossRef]
- Dunn, K.; Aotaki-Keen, A.; Putkey, F.; Hjelmeland, L. ARPE-19, A Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef]
- Paul, D. Elaborations on the Higuchi Model for Drug Delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Mulye, N.V.; Turco, S.J. A Simple Model Based on First Order Kinetics to Explain Release of Highly Water Soluble Drugs from Porous Dicalcium Phosphate Dihydrate Matrices. Drug Dev. Ind. Pharm. 1995, 21, 943–953. [Google Scholar] [CrossRef]
- Malvern Panalytical. Using a Rotational Rheometer to Assess Adhesion and Tackiness. Available online: https://www.azom.com/article.aspx?ArticleID=12166 (accessed on 30 July 2019).








| NPs | Total Concentration (µg/mL) | EE (%) | DL (%) |
|---|---|---|---|
| Crocin | 1200 | 89.8 ± 0.1 | 29.927 ± 0.038 |
| 600 | 74.9 ± 1.0 | 11.228 ± 0.149 | |
| 200 | 78.8 ± 0.2 | 3.939 ± 0.004 | |
| 40 | 23.5 ± 2.7 | 0.235 ± 0.027 | |
| 20 | 44.4 ± 3.8 | 0.222 ± 0.019 | |
| 4 | 91.7 ± 2.7 | 0.092 ± 0.003 | |
| ACT | 40 | 2.6 ± 8.2 | 0.026 ± 0.082 |
| 20 | 4.8 ± 3.4 | 0.024 ± 0.017 | |
| 4 | 5.6 ± 40.4 | 0.006 ± 0.040 |
| NPs | Total Concentration (µg/mL) | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| Empty NPs | 0 | 323.4 ± 9.9 | 0.186 ± 0.029 | +37.7 ± 1.1 |
| Crocin | 1200 | 295.3 ± 1.1 | 0.174 ± 0.020 | +38.8 ± 0.8 |
| 600 | 309.2 ± 4.4 | 0.193 ± 0.006 | +37.6 ± 0.4 | |
| 200 | 304.1 ± 3.1 | 0.196 ± 0.008 | +38.9 ± 0.9 | |
| 40 | 296.5 ± 5.1 | 0.179 ± 0.020 | +33.2 ± 0.9 | |
| 20 | 302.4 ± 5.1 | 0.177 ± 0.015 | +35.0 ± 1.2 | |
| 4 | 292.4 ± 2.9 | 0.142 ± 0.030 | +36.0 ± 2.2 | |
| ACT | 40 | 316.5 ± 2.5 | 0.196 ± 0.009 | +36.6 ± 0.5 |
| 20 | 328.9 ± 4.0 | 0.235 ± 0.017 | +36.8 ± 0.8 | |
| 4 | 318.6 ± 1.7 | 0.189 ± 0.025 | +37.0 ± 0.7 | |
| Crocin + ACT | 600 + 40 | 330.1 ± 5.5 | 0.232 ± 0.013 | +41.0 ± 0.4 |
| Models | Parameters | Free Crocin | NPs + Crocin |
|---|---|---|---|
| Zero order | R2adj | 0.680 | 0.770 |
| AIC | 46.907 | 43.275 | |
| k0 (µg/h) | 26.212 | 20.546 | |
| First order | R2adj | 0.801 | 0.855 |
| AIC | 44.277 | 39.479 | |
| k1 (h−1) | 0.743 | 0.407 | |
| Higuchi | R2adj | 0.870 | 0.801 |
| AIC | 41.941 | 43.081 | |
| kH (h−0.5) | 50.203 | 38.853 | |
| Korsmeyer-Peppas | R2adj | 0.779 | 0.820 |
| AIC | 44.199 | 42.753 | |
| kKP (hn) | 48.473 | 29.720 | |
| N | 0.576 | 0.731 |
| Samples | Peak Normal Force-Normal Force (N) | Area under Force Time Curve (N·s) |
|---|---|---|
| Empty NPs | −0.132 ± 0.006 | 0.572 ± 0.428 |
| NPs + crocin (600 µg/mL) | −0.178 ± 0.018 | 0.507 ± 0.081 |
| NPs + ACT (40 µg/mL) | −0.172 ± 0.006 | 0.416 ± 0.100 |
| NPs + crocin (600 µg/mL) + ACT (40 µg/mL) | −0.178 ± 0.012 | 0.438 ± 0.085 |
| Mucin | −0.166 ± 0.006 | 0.414 ± 0.061 |
| Empty NPs + Mucin | −0.162 ± 0.009 | 0.469 ± 0.047 |
| NPs + crocin (600 µg/mL) + Mucin | −0.167 ± 0.008 | 0.422 ± 0.058 |
| NPs + ACT (40 µg/mL) + Mucin | −0.169 ± 0.005 | 0.466 ± 0.069 |
| NPs + crocin (600 µg/mL) + ACT (40 µg/mL) + Mucin | −0.165 ± 0.003 | 0.479 ± 0.103 |
| Sample | ZP (mV) |
|---|---|
| Empty NPs | +34.1 ± 1.9 |
| NPs + crocin (600 µg/mL) | +39.2 ± 1.3 |
| NPs + ACT (40 µg/mL) | +41.4 ± 2.0 |
| NPs + crocin (600 µg/mL) + ACT (40 µg/mL) | +40.2 ± 1.6 |
| Mucin | −7.0 ± 0.2 |
| Empty NPs + Mucin | −9.7 ± 1.1 |
| NPs + crocin (600 µg/mL) + Mucin | −9.3 ± 0.3 |
| NPs + ACT (40 µg/mL) + Mucin | −10.4 ± 0.6 |
| NPs + crocin (600 µg/mL) + ACT (40 µg/mL) + Mucin | −9.3 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, S.; Silva, B.; Martins, A.M.; Ribeiro, H.M.; Gonçalves, L.; Marto, J. Antioxidant-Loaded Mucoadhesive Nanoparticles for Eye Drug Delivery: A New Strategy to Reduce Oxidative Stress. Processes 2021, 9, 379. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020379
Cordeiro S, Silva B, Martins AM, Ribeiro HM, Gonçalves L, Marto J. Antioxidant-Loaded Mucoadhesive Nanoparticles for Eye Drug Delivery: A New Strategy to Reduce Oxidative Stress. Processes. 2021; 9(2):379. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020379
Chicago/Turabian StyleCordeiro, Sandra, Beatriz Silva, Ana Margarida Martins, Helena Margarida Ribeiro, Lídia Gonçalves, and Joana Marto. 2021. "Antioxidant-Loaded Mucoadhesive Nanoparticles for Eye Drug Delivery: A New Strategy to Reduce Oxidative Stress" Processes 9, no. 2: 379. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020379