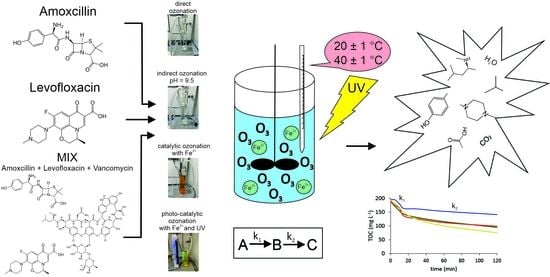
Fe2+ and UV Catalytically Enhanced Ozonation of Selected Environmentally Persistent Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Antibiotics
2.2. Thermal Stability Testing
2.3. Experimental Set-Up
2.4. Degradation Kinetics
3. Results and Discussion
3.1. Model Antibiotics
3.2. Ozonation
3.2.1. Ozonation
3.2.2. Indirect Ozonation
3.3. Catalytic Ozonation
3.3.1. Ozonation with Fe2+
3.3.2. Ozonation with Fe2+ and UV
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ay, F.; Kargi, F. Advanced Oxidation of Amoxicillin by Fenton’s Reagent Treatment. J. Hazard. Mater. 2010, 179, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Alaton, I.; Dogruel, S. Pre-Treatment of Penicillin Formulation Effluent by Advanced Oxidation Processes. J. Hazard. Mater. 2004, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Ciniglia, C.; De Champdoré, M.; Lo Giudice, R.; Marotta, R.; Zuccato, E. Antibiotics in the Environment: Occurrence in Italian STPs, Fate, and Preliminary Assessment on Algal Toxicity of Amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef]
- Homem, V.; Santos, L. Degradation and Removal Methods of Antibiotics from Aqueous Matrices—A Review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Elmolla, E.; Chaudhuri, M. Optimization of Fenton Process for Treatment of Amoxicillin, Ampicillin and Cloxacillin Antibiotics in Aqueous Solution. J. Hazard. Mater. 2009, 170, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kairigo, P.; Ngumba, E.; Sundberg, L.R.; Gachanja, A.; Tuhkanen, T. Occurrence of Antibiotics and Risk of Antibiotic Resistance Evolution in Selected Kenyan Wastewaters, Surface Waters and Sediments. Sci. Total Environ. 2020, 720, 137580. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the Concentrations and Risks of Toxicity from the Antibiotics Ciprofloxacin, Sulfamethoxazole, Trimethoprim and Erythromycin in European Rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Yargeau, V.; Leclair, C. Potential of Ozonation for the Degradation of Antibiotics in Wastewater. Water Sci. Technol. 2007, 55, 321–326. [Google Scholar] [CrossRef]
- Adelglass, J.; DeAbate, C.A.; McElvaine, P.; Fowler, C.L.; LoCocco, J.; Campbell, T. Comparison of the Effectiveness of Levofloxacin and Amoxicillin- Clavulanate for the Treatment of Acute Sinusitis in Adults. Otolaryngol. Head Neck Surg. 1999, 120, 320–327. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhang, Z.; Li, P.; Zang, Y.; Liu, X. Antibiotics in Aquatic Environments of China: A Review and Meta-Analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C. Ecotoxicological Aspects Related to the Presence of Pharmaceuticals in the Aquatic Environment. J Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, N.; Yasojima, M.; Nakada, N.; Miyajima, K.; Komori, K.; Suzuki, Y.; Tanaka, H. Effects of Antibacterial Agents, Levofloxacin and Clarithromycin, on Aquatic Organisms. Water Sci. Technol. 2006, 53, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of Antibiotic Residues in Various Environmental Compartments of Shandong Province in Eastern China: Its Potential for Resistance Development and Ecological and Human Risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef]
- Cao, M.; Feng, Y.; Zhang, Y.; Kang, W.; Lian, K.; Ai, L. Studies on the Metabolism and Degradation of Vancomycin in Simulated in Vitro and Aquatic Environment by UHPLC-Triple-TOF-MS/MS. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshian, J.; Dahdaleh, N.S.; Lam, S.K.; Savage, J.W.; Smith, Z.A. The Use of Vancomycin Powder in Modern Spine Surgery: Systematic Review and Meta-Analysis of the Clinical Evidence. World Neurosurg. 2015, 83, 816–823. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, Occurrence and Fate of Antibiotics in the Italian Aquatic Environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Havelkova, B.; Beklova, M.; Kovacova, V.; Hlavkova, D.; Pikula, J. Ecotoxicity of Selected Antibiotics for Organisms of Aquatic and Terrestrial Ecosystems. Neuroendocr. Lett. 2016, 37, 38–44. [Google Scholar]
- Chen, Y.; Xie, Y.; Yang, J.; Cao, H.; Zhang, Y. Reaction Mechanism and Metal Ion Transformation in Photocatalytic Ozonation of Phenol and Oxalic Acid with Ag+/TiO2. J. Environ. Sci. 2014, 26, 662–672. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of Antibiotics by Advanced Oxidation Processes: An Overview. Sci. Total Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, J.; Kasprzyk-Hordern, B. The Efficiency and Mechanisms of Catalytic Ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Anjali, R.; Shanthakumar, S. Insights on the Current Status of Occurrence and Removal of Antibiotics in Wastewater by Advanced Oxidation Processes. J. Environ. Manag. 2019, 246, 51–62. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater- A Review. Chem. Eng. J. Adv. 2020, 3. [Google Scholar] [CrossRef]
- Boševski, I.; Kalčikova, G.; Cerkovnik, J.; Žgajnar Gotvajn, A. Ozone as a Pretreatment Method for Antibiotic Contaminated Wastewater and Sludge. Ozone Sci. Eng. 2020, 42, 128–135. [Google Scholar] [CrossRef]
- Mansour, S.; Knani, S.; Bensouilah, R.; Ksibi, Z. Wastewater Problems and Treatments. Curr. Trends Futur. Dev. Membr. Membr. Environ. Appl. 2019, 151–174. [Google Scholar] [CrossRef]
- Kıdak, R.; Doğan, Ş. Medium-High Frequency Ultrasound and Ozone Based Advanced Oxidation for Amoxicillin Removal in Water. Ultrason. Sonochem. 2018, 40, 131–139. [Google Scholar] [CrossRef]
- Marcelino, R.B.P.; Leão, M.M.D.; Lago, R.M.; Amorim, C.C. Multistage Ozone and Biological Treatment System for Real Wastewater Containing Antibiotics. J. Environ. Manag. 2017, 195, 110–116. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Rodayan, A.; Berk, D.; Yargeau, V. Removal of the Antibiotic Levofloxacin (LEVO) in Water by Ozonation and TiO2 Photocatalysis. Chem. Eng. J. 2012, 189–190, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Dodd, M.C.; Buffle, M.O.; Von Gunten, U. Oxidation of Antibacterial Molecules by Aqueous Ozone: Moiety-Specific Reaction Kinetics and Application to Ozone-Based Wastewater Treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar] [CrossRef]
- Golet, E.M.; Alder, A.C.; Hartmann, A.; Ternes, T.A.; Giger, W. Trace Determination of Fluoroquinolone Antibacterial Agents in Urban Wastewater by Solid-Phase Extraction and Liquid Chromatography with Fluorescence Detection. Anal. Chem. 2001, 73, 3632–3638. [Google Scholar] [CrossRef]
- Andreozzi, R.; Canterino, M.; Marotta, R.; Paxeus, N. Antibiotic Removal from Wastewaters: The Ozonation of Amoxicillin. J. Hazard. Mater. 2005, 122, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Žgajnar Gotvajn, A.; Derco, J.; Vrabeľ, M.; Kassai, A. Improvement of Biotreatability of Environmentally Persistent Antibiotic Tiamulin by O3 and O3/H2O2 Oxidation Processes. Environ. Technol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oxidationtech. Fundementals of Ozone Solubility. Available online: https://www.oxidationtech.com/ozone/solubility/fundementals-of-ozone-solubility.html (accessed on 4 January 2021).
- Beltrán, F.J. Ozone Reaction Kinetics for Water and Wastewater System; Lewis Publishers: Boca Raton, FL, USA, 2004; ISBN 9781135463076. [Google Scholar]
- Mojiri, A.; Vakili, M.; Farraji, H.; Aziz, S.Q. Combined Ozone Oxidation Process and Adsorption Methods for the Removal of Acetaminophen and Amoxicillin from Aqueous Solution; Kinetic and Optimisation. Environ. Technol. Innov. 2019, 15, 100404. [Google Scholar] [CrossRef]
- De Witte, B.; Van Langenhove, H.; Hemelsoet, K.; Demeestere, K.; De Wispelaere, P.; Van Speybroeck, V.; Dewulf, J. Levofloxacin Ozonation in Water: Rate Determining Process Parameters and Reaction Pathway Elucidation. Chemosphere 2009, 76, 683–689. [Google Scholar] [CrossRef]
- Lofrano, G.; Carotenuto, M.; Uyguner-Demirel, C.S.; Vitagliano, A.; Siciliano, A.; Guida, M. An Integrated Chemical and Ecotoxicological Assessment for the Photocatalytic Degradation of Vancomycin. Environ. Technol. 2014, 35, 1234–1242. [Google Scholar] [CrossRef] [Green Version]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, Mineralization and Antibiotic Inactivation of Amoxicillin by UV-A/TiO 2 Photocatalysis. J. Environ. Manag. 2012, 98, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Kansal, S.K.; Kundu, P.; Sood, S.; Lamba, R.; Umar, A.; Mehta, S.K. Photocatalytic Degradation of the Antibiotic Levofloxacin Using Highly Crystalline TiO2 Nanoparticles. New J. Chem. 2014, 38, 3220–3226. [Google Scholar] [CrossRef]
- Kaur, M.; Umar, A.; Mehta, S.K.; Kansal, S.K. Reduced Graphene Oxide-CdS Heterostructure: An Efficient Fluorescent Probe for the Sensing of Ag(I) and Sunset Yellow and a Visible-Light Responsive Photocatalyst for the Degradation of Levofloxacin Drug in Aqueous Phase. Appl. Catal. B Environ. 2019, 245, 143–158. [Google Scholar] [CrossRef]



| Treatment Method | Ozonation Setting | Buffer Solution | pH at t = 0 min [/] | Concentration of Fe2+ [mmol·L−1] | UV |
|---|---|---|---|---|---|
| Direct ozonation (O3) | Ø = 30 L·h−1 γ = 0.1 g·L−1 | / | <3.0 | / | / |
| Indirect ozonation (O3/pH9.5) | Ø = 30 L·h−1 γ = 0.1 g·L−1 | ✓ | 9.5 ± 0.2 | / | / |
| Catalytic ozonation (O3/Fe2+) | Ø = 30 L·h−1 γ = 0.1 g·L−1 | / | <3.0 | 1.0 | / |
| Photocatalytic ozonation (O3/Fe2+/UV) | Ø = 30 L·h−1 γ = 0.1 g·L−1 | / | <3.0 | 1.0 | ✓ |
| Antibiotic | Concentration [mg·L−1] | TOC [mg·L−1] | COD [mg·L−1] |
|---|---|---|---|
| Amoxicillin (AM) | 400 | 209.4 ± 0.8 | 598.5 ± 1.2 |
| Levofloxacin (LV) | 400 | 233.9 ± 0.7 | 575.6 ± 26.4 |
| The mixture (MIX) | 100 AM + 100 LV + 100 VM | 152.3 ± 1.4 | 428.0 ± 32.0 |
| Treatment Efficiency [%] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| Phase of ozonation | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Amoxicillin (AM) | 9.8 ± 1.2 | 27.7 ± 5.3 | 15.7 | 40.8 | 38.3 ± 1.6 | 60.6 ± 3.1 | 49.7 | 68.5 |
| Levofloxacin (LV) | 16.2 ± 1.6 | 38.5 ± 2.5 | 10.9 | 56.4 | 42.7 ± 12.7 | 67.2 ± 3.6 | 41.9 | 75.1 |
| The mixture (MIX) | 19.7 ± 0.2 | 36.4 ± 3.4 | 32.0 | 60.4 | 48.2 ± 0.0 | 67.2 ± 1.8 | 38.0 | 75.7 |
| Rate Constants [min−1] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| k1 | k2 | k1 | k2 | k1 | k2 | k1 | k2 | |
| Amoxicillin (AM) | 0.0125 | 0.0015 | 0.0117 | 0.0031 | 0.0352 | 0.0033 | 0.0462 | 0.0041 |
| Levofloxacin (LV) | 0.0186 | 0.0017 | 0.0080 | 0.0061 | 0.0474 | 0.0036 | 0.0336 | 0.0072 |
| The mixture (MIX) | 0.0163 | 0.0016 | 0.0240 | 0.0046 | 0.0447 | 0.0019 | 0.0324 | 0.0077 |
| Arrhenius Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Phase of Ozonation | 1st | 2nd | 1st | 2nd | ||||
| A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | |
| Amoxicillin (AM) | 0 | / | 234.3 | 29,231 | 3.11 | 10,950 | 0.12 | 8741 |
| Levofloxacin (LV) | 0 | / | 2.35 × 106 | 51,447 | 4.87 × 1012 | 78,862 | 328 | 27,911 |
| The mixture (MIX) | 2.84 × 1017 | 108,297 | 1.71 × 1021 | 135,242 | 0 | / | 1.95 × 107 | 56,348 |
| Treatment Efficiency [%] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| Phase of ozonation | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Amoxicillin (AM) | 21.1 ± 3.4 | 49.5 ± 4.7 | 36.6 | 73.5 | 56.2 ± 3.5 | 86.6 ± 1.3 | 69.2 | 91.9 |
| Levofloxacin (LV) | 23.6 ± 0.7 | 60.8 ± 8.6 | 31.4 | 78.1 | 53.3 ± 3.1 | 79.5 ± 6.1 | 60.4 | 92.1 |
| The mixture (MIX) | 27.7 ± 7.1 | 51.6 ± 4.2 | 33.7 | 73.6 | 55.4 ± 13.5 | 79.6 ± 1.6 | 56.2 | 93.4 |
| Rate Constants [min−1] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| k1 | k2 | k1 | k2 | k1 | k2 | k1 | k2 | |
| Amoxicillin (AM) | 0.0187 | 0.0035 | 0.0302 | 0.0082 | 0.0562 | 0.0096 | 0.0820 | 0.0097 |
| Levofloxacin (LV) | 0.0182 | 0.0047 | 0.0260 | 0.0101 | 0.0517 | 0.0042 | 0.0605 | 0.0070 |
| The mixture (MIX) | 0.0194 | 0.0028 | 0.0263 | 0.0080 | 0.0488 | 0.0043 | 0.0528 | 0.0142 |
| Arrhenius Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Phase of Ozonation | 1st | 2nd | 1st | 2nd | ||||
| A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | |
| Amoxicillin (AM) | 50.24 | 19,301 | 4317 | 34,282 | 28.36 | 15,213 | 0.011 | 417.3 |
| Levofloxacin (LV) | 6.48 | 14,362 | 1397 | 30,804 | 0.69 | 6329 | 18.96 | 20,569 |
| The mixture (MIX) | 2.92 | 12,253 | 90,790 | 42,273 | 0.18 | 3172 | 1.5·106 | 48,104 |
| Treatment Efficiency [%] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| Phase of ozonation | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Amoxicillin (AM) | 17.0 ± 3.3 | 46.6 ± 14.7 | 45.1 | 82.7 | 47.6 ± 15.9 | 85.8 ± 5.7 | 45.1 | 82.8 |
| Levofloxacin (LV) | 7.0 ± 1.8 | 50.7 ± 1.2 | 14.5 | 60.9 | 45.1 ± 7.4 | 81.9 ± 0.3 | 44.7 | 87.6 |
| The mixture (MIX) | 15.5 ± 6.3 | 46.7 ± 1.1 | 30.1 | 62.8 | 51.4 ± 19.2 | 79.9 ± 1.1 | 54.7 | 77.4 |
| Rate Constants [min−1] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| k1 | k2 | k1 | k2 | k1 | k2 | k1 | k2 | |
| Amoxicillin (AM) | 0.0211 | 0.0026 | 0.0293 | 0.0028 | 0.0534 | 0.0092 | 0.0385 | 0.0063 |
| Levofloxacin (LV) | 0.0181 | 0.0038 | 0.0113 | 0.0055 | 0.0437 | 0.0089 | 0.0399 | 0.0086 |
| The mixture (MIX) | 0.0228 | 0.0032 | 0.0231 | 0.0047 | 0.0605 | 0.0041 | 0.0514 | 0.0027 |
| Arrhenius Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Phase of Ozonation | 1st | 2nd | 1st | 2nd | ||||
| A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | |
| Amoxicillin (AM) | 4.71 | 13,220 | 0.0088 | 2984 | 0 | / | 1.80·10−5 | / |
| Levofloxacin (LV) | 0 | / | 1.68 | 14,889 | 0.01 | / | 0.005 | / |
| The mixture (MIX) | 0.03 | 526 | 1.80 | 15,479 | 0 | / | 4.21·10−6 | / |
| Treatment Efficiency [%] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| Phase of ozonation | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Amoxicillin (AM) | 23.4 ± 0.0 | 60.7 ± 8.4 | 51.5 | 84.6 | 57.3 ± 0.4 | 85.4 ± 3.6 | 51.5 | 84.6 |
| Levofloxacin (LV) | 12.2 ± 4.8 | 59.2 ± 5.2 | 11.4 | 58.0 | 39.3 ± 5.1 | 86.0 ± 6.2 | 64.3 | 87.5 |
| The mixture (MIX) | 13.0 ± 7.1 | 64.9 ± 0.6 | 8.1 | 71.5 | 28.3 ± 8.5 | 85.3 ± 2.5 | 34.5 | 89.8 |
| Rate Constants [min−1] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Temperature [°C] | 21 ± 1 | 40 ± 1 | 21 ± 1 | 40 ± 1 | ||||
| k1 | k2 | k1 | k2 | k1 | k2 | k1 | k2 | |
| Amoxicillin (AM) | 0.0176 | 0.0054 | 0.0146 | 0.0025 | 0.0573 | 0.0085 | 0.0487 | 0.0068 |
| Levofloxacin (LV) | 0.0056 | 0.0084 | 0.0090 | 0.0057 | 0.0328 | 0.0116 | 0.0679 | 0.0099 |
| The mixture (MIX) | 0.0054 | 0.0069 | 0.0052 | 0.0084 | 0.0326 | 0.0074 | 0.0282 | 0.0135 |
| Arrhenius Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| TOC | COD | |||||||
| Phase of Ozonation | 1st | 2nd | 1st | 2nd | ||||
| A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | A [min−1] | Ea [J·mol−1] | |
| Amoxicillin (AM) | 0 | / | 1.67 × 10−8 | / | 0 | / | 0.000215 | / |
| Levofloxacin (LV) | 13.89 | 19,105 | 1.41 × 10−5 | / | 5266 | 29,298 | 0.00085 | / |
| The mixture (MIX) | 0 | / | 0.18 | 7921 | 0 | / | 148.1 | 24,209 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gotvajn, A.Ž.; Rozman, U.; Antončič, T.; Urbanc, T.; Vrabeľ, M.; Derco, J. Fe2+ and UV Catalytically Enhanced Ozonation of Selected Environmentally Persistent Antibiotics. Processes 2021, 9, 521. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9030521
Gotvajn AŽ, Rozman U, Antončič T, Urbanc T, Vrabeľ M, Derco J. Fe2+ and UV Catalytically Enhanced Ozonation of Selected Environmentally Persistent Antibiotics. Processes. 2021; 9(3):521. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9030521
Chicago/Turabian StyleGotvajn, Andreja Žgajnar, Ula Rozman, Teja Antončič, Teja Urbanc, Martin Vrabeľ, and Ján Derco. 2021. "Fe2+ and UV Catalytically Enhanced Ozonation of Selected Environmentally Persistent Antibiotics" Processes 9, no. 3: 521. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9030521