Infrared Irradiation Drying Impact on Bee Pollen: Case Study on the Phenolic Composition of Eucalyptus globulus Labill and Salix atrocinerea Brot. Pollens
Abstract
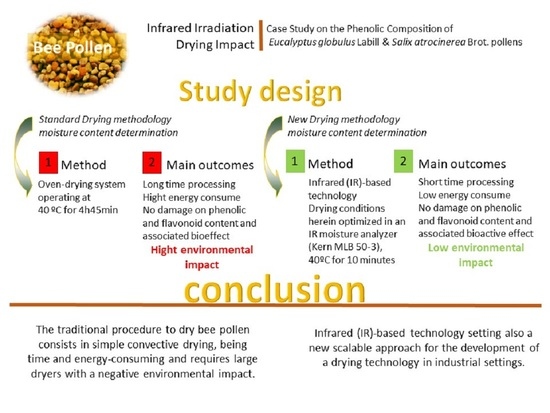
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bee Pollen Samples
2.3. Drying Process
2.4. Moisture Determination
2.5. Extracts Preparation
2.6. Chromatographic Analysis
2.7. Free Radical Scavenging Activity
2.8. Statistical Analysis
3. Results
3.1. Water Content Determination
3.2. Infrared Drying Process
3.3. Pollen Phenolic/Flavonoid Profile
3.4. IR Radiation Effect on Phenolic Composition and on the Radical Scavenger Bioactivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- EFSA Supporting Pub. Technical Report. EN-1234. 2017; Volume 14. Available online: https://0-efsa-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/abs/10.2903/sp.efsa.2017.EN-1234 (accessed on 1 April 2021). [CrossRef]
- Campos, M.G.; Bogdanov, S.; Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Kroyer, G.; Hegedus, N. Evaluation of bioactive properties of pollen extracts as functional dietary food supplement. Innov. Food Sci. Emerg. Technol. 2001, 2, 171–174. [Google Scholar] [CrossRef]
- Mărgăoan, R.; Mărghitas, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.A.; Bobiş, O. Predominant and secondary pollen botanical origins influence the carotenoid and fatty acid profile in fresh honeybee-collected pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, F.; Encarnação, T.; Campos, M.G. Algae as functional foods for the elderly. Food Nut. Sci. 2016, 7, 1122–1148. [Google Scholar] [CrossRef] [Green Version]
- Peris, M.J. Producción y comércio de los produtos apícolas en España. 1º Congreso Nacional de Apicultura (Madrid). El Campo del Banco de Bilbao. Apicultura 1984, 93, 40–68. [Google Scholar]
- Orzaez-Villanueva, M.T.; Diaz-Marquina, A.; Bravo-Serrano, R.; Blazquez-Abellan, G. The importance of bee-collected pollen in the diet: A study of its composition. Int. J. Food Sci. Nut. 2002, 53, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.A.; Mareczek, G.; Wyżgolik, J.; Klepacz-Baniak, K.C. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Pascoal, A.; Rodrigues, S.; Teixeira, A.; Feásc, X.; Estevinho, L.M. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxic. 2014, 63, 233–239. [Google Scholar] [CrossRef]
- Silva, D.N.A.; João, J.S.S.; Campos, M.G. Biological and Functional Properties of Bee Products for Medicinal Purposes. In Traditional and Folk Herbal Medicine: Recent Researches, 1st ed.; Gupta, V.K., Ed.; Daya Publishing House: New Delhi, India, 2014; Chapter 18; pp. 541–562, ISBN-10: 8170358744. [Google Scholar]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee pollen and bee bread: Bioactive constituents and health benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Anjos, O.; Paula, V.; Delgado, T.; Estevinho, L. Influence of the storage conditions on the quality of bee pollen. Zemdirb. Agric. 2019, 106, 87–94. [Google Scholar] [CrossRef]
- Jagdis, A.; Sussman, G. Anaphylaxis from bee pollen supplement. Canadian Med. Assoc. J. 2012, 184, 1167–1169. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Genetically Modified Organisms (GMO). Statement on the safety of MON810 maize pollen occurring in or as food. panel on genetically modified organisms (GMO). EFSA J. 2011, 11, 2434. [Google Scholar]
- EFSA. Scientific opinion on an application (EFSA-GMO-NL-2012-107) for the placing on the market of maize MON 810 pollen under Regulation (EC) No 1829/2003 from Monsanto. EFSA J. 2012, 10, 3022. [Google Scholar] [CrossRef] [Green Version]
- Szczesna, T.; Rybak-Chimielewska, H.; Chmielewsky, W. Sugar composition of pollen loads harvested at different periods of the beekeeping season. J. Apic. Sci. 2002, 46, 107–115. [Google Scholar]
- Campos, M.G. Caracterização do Pólen Apícola Pelo Seu Perfil em Compostos Fenólicos e Pesquisa de Algumas Actividades Biologicas. Ph.D. Thesis, Faculty of Pharmacy, University of Coimbra, Coimbra, Portugal, 1997. [Google Scholar]
- Campos, M.G.; Mitchel, K.; Cunha, A.; Markham, K.R. A systematic approach to the characterisation of bee pollens via their flavonoid/phenolic profiles. Phytochem. Anal. 1997, 8, 181–185. [Google Scholar] [CrossRef]
- Campos, M.G.; Webby, R.F.; Markham, K.R.; Mitchell, K.A.; Cunha, A.P. Age-induced diminution of free radical scavenging capacity in bee pollens and the contribution of constituent flavonoids. J. Agric. Food Chem. 2003, 51, 742–745. [Google Scholar] [CrossRef] [Green Version]
- Bakour, M.; Campos, M.G.; Imtara, H.; Lyoussi, B. Antioxidant content and identification of phenolic/flavonoid compounds in the pollen of fourteen plants using HPLC-DAD. J. Apic. Res. 2020, 59, 35–41. [Google Scholar] [CrossRef]
- Bovi, T.S.; Caeiro, A.; Santos, S.A.; Zaluski, R.; Shinohara, A.J.; Lima, G.P.P.; Campos, M.G.; Junior, A.J.; Orsi, R.O. Flavonoid content in bee bread suffers seasonal variation and affects hypopharyngeal gland development in Apis mellifera honey bees. J. Apic. Res. 2019, 20, 1–8. [Google Scholar]
- Negri, G.; Barreto, L.M.R.C.; Sper, F.L.; Carvalho, C.D.; Campos, M.G. Phytochemical analysis and botanical origin of Apis mellifera bee pollen from the municipality of Canavieiras, Bahia State, Brazil. Braz. J. Food Techn. 2018, 21, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Urcan, A.C.; Criste, A.D.; Dezmirean, D.S.; Mărgăoan, R.; Caeiro, A.; Campos, M.G. Similarity of data from bee bread with the same taxa collected in India and Romania. Molecules 2018, 23, 2491. [Google Scholar] [CrossRef] [Green Version]
- Infarmed-National Authority of Medicines and Health Products. I.P. Perda Por Secagem. In Portuguese Pharmacopoeia, 7th ed; Infarmed, Ed.; Imprensa Nacional: Lisboa, Portugal, 2005; Chapter 2.2.32.d; pp. 49–50. [Google Scholar]
- Campos, M.G.; Markham, K.R. Structure Information from HPLC and on-Line Measured Absorption Spectra: Flavones, Flavonols and Phenolic Acids, 1st ed.; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2007; pp. 14, 26–29, 91, 104. ISBN 978-989-8074-05-8. [Google Scholar]
- Almaraz-Abarca, N.; Campos, M.G.; Delgado-Alvarado, E.A.; Ávila-Reyes, J.A.; Herrera-Corral, J.; González-Valdez, L.S.; Naranjo-Jiménez, N.; Frigerio, C.; Tomatas, A.F.; Almeida, A.J.; et al. Pollen flavonoid/phenolic acid composition of four species of cactaceae and its taxonomic significance. Am. J. Agric. Biol. Sci. 2008, 3, 534–543. [Google Scholar]
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Rebiai, A.; Lanez, T. Chemical composition and antioxidant activity of Apis mellifera bee pollen from northwest Algeria. J. Fund. Appl. Sci. 2012, 4, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Šaric, A.; Balog, T.; Sobočanec, S.; Kušić, B.; Šverko, V.; Rusak, G.; Likić, S.; Bubalo, D.; Pinto, B.; Reali, D.; et al. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem. Toxic. 2009, 47, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Comp. Anal. 2005, 18, 106–111. [Google Scholar] [CrossRef]
- Conte, G.; Bednelli, G.; Serra, A.; Signorini, F.; Bientinesi, M.; Nicolella, C.; Mele, M.; Canale, M. Lipid characterization of chestnut and willow honeybee-collectged pollen: Impact of freeze-drying and microwave-assisted drying. J. Food Comp. Anal. 2016. [Google Scholar] [CrossRef]
- Gardana, C.; Del Bo’, C.; Quicazán, M.C.; Correa, A.R.; Simonetti, P. Nutrients, phytochemicals and botanical origin of commercial bee pollen from different geographical areas. J. Food Comp. Anal. 2018, 73, 29–38. [Google Scholar] [CrossRef]
- Bedlina-Aldemita, D.; Opper, C.; Schreiner, M.; D’Amico, S. Nutritional composition of pot-pollen produced by stingless bees (Tetragona biroi Friese) from the Philippines. J. Food Comp. Anal. 2019, 82, 103215. [Google Scholar] [CrossRef]
- Medina, A.; Gonzalez, G.; Saez, J.M.; Mateo, R.; Jimenez, M. Bee pollen, a substrate that stimulates Ochratoxin A production by Aspergillus ochraceus Wilh. Syst. Appl. Microb. 2004, 27, 261–267. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Font, G.; Mañes, J.; Berrada, H. Determination of mycotoxins in bee pollen by gas chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2013, 61, 1999–2005. [Google Scholar] [CrossRef]
- Melo, I.; Almeida-Muradian, L. Comparison of methodologies for moisture determination on dried bee pollen samples. Food Sci. Techn. 2011, 31, 194–197. [Google Scholar] [CrossRef] [Green Version]
- Arruda, V.A.S.; Pereira, A.A.S.; de Freitas, A.S.; Barth, O.M.; Almeida-Muradian, L.B. Dried bee pollen: B complex vitamins, physicochemical and botanical composition. J. Food Comp. Anal. 2013, 29, 100–105. [Google Scholar] [CrossRef]
- Isik, A.; Ozdemir, M.; Doymaz, I. Infrared drying of bee pollen: Effects and impacts on food components. Czech J. Food Sci. 2019, 37, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Isik, A.; Ozdemir, M.; Doymaz, I. Effect of hot air drying on quality characteristics and physicochemical properties of bee pollen. Food Sci. Tech. 2019, 39, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Kanar, Y.; Mazi, B.G. Effect of different drying methods on antioxidant characteristics of bee-pollen. J. Food Measur. Charact. 2019, 13, 3376–3386. [Google Scholar] [CrossRef]
- Keskin, M.; Özkök, A. Effects of drying techniques on chemical composition and volatile constituents of bee pollen. Czech J. Food Sci. 2020, 38, 203–208. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Piao, J.P.; Park, C.H.; Cho, H.D. Far infrared irradiation enhances nutraceutical compounds and antioxidant properties in Angelica gigas nakai powder. Antioxidants 2018, 7, 189. [Google Scholar] [CrossRef] [Green Version]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobiş, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In-vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Pilawa, B.; Ramos, P.; Stojko, J. Antioxidative properties of bee pollen extracts examined by EPR spectroscopy. J. Apic. Sci. 2012, 56, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.M.S.; Câmara, C.A.; Silva Lins, A.C.; Barbosa-Filho, J.M.; Freitas da Silva, E.M.S.; Santos, F.B.M. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J. Food Comp. Anal. 2006, 19, 507–511. [Google Scholar] [CrossRef]






| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |
|---|---|---|---|---|---|
| 11.79 | 27.82 | 26.1 | 2.26 | 22.34 | |
| 11.48 | 27.79 | 26.1 | 1.3 | 23.94 | |
| 11.08 | 27.88 | 25.88 | 2.19 | 22.05 | |
| 11.49 | 27.48 | 25.09 | 2.48 | 22.69 | |
| 11.61 | 27.86 | 25.86 | 2.08 | 22.79 | |
| Std deviation | 0.26 | 0.16 | 0.42 | 0.17 | 0.34 |
| Average | 11.49 | 27.77 | 25.81 | 2.25 | 22.47 |
| RSD% | 2.27 | 0.59 | 1.61 | 7.49 | 1.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, M.G.; Frigerio, C.; Bobiş, O.; Urcan, A.C.; Gomes, N.G.M. Infrared Irradiation Drying Impact on Bee Pollen: Case Study on the Phenolic Composition of Eucalyptus globulus Labill and Salix atrocinerea Brot. Pollens. Processes 2021, 9, 890. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050890
Campos MG, Frigerio C, Bobiş O, Urcan AC, Gomes NGM. Infrared Irradiation Drying Impact on Bee Pollen: Case Study on the Phenolic Composition of Eucalyptus globulus Labill and Salix atrocinerea Brot. Pollens. Processes. 2021; 9(5):890. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050890
Chicago/Turabian StyleCampos, Maria G., Christian Frigerio, Otilia Bobiş, Adriana C. Urcan, and Nelson G. M. Gomes. 2021. "Infrared Irradiation Drying Impact on Bee Pollen: Case Study on the Phenolic Composition of Eucalyptus globulus Labill and Salix atrocinerea Brot. Pollens" Processes 9, no. 5: 890. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050890