Clear Cell Acanthoma: A Review of Clinical and Histologic Variants
Abstract
:1. Introduction
2. Material and Methods
2.1. Case 1
2.2. Case 2
3. Discussion
3.1. Variants of CCA
3.1.1. Giant CCA
3.1.2. Mucosal CCA
3.1.3. Verrucous CCA
3.1.4. Polypoid CCA
3.1.5. Atypical CCA
3.1.6. Pigmented CCA
3.1.7. Eruptive CCA
3.1.8. Cystic CCA
3.1.9. Linear CCA
3.1.10. Subungual CCA
3.1.11. Trichilemmal CCA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Degos, R.; Delort, J.; Civatte, J.; Poiares Baptista, A. Epidermal tumor with an unusual appearance: Clear cell acanthoma. Ann. Dermatol. Syphiligr. 1962, 89, 361–371. [Google Scholar]
- Degos, R.; Civatte, J. Clear-cell acanthoma* Experience of 8 years. Br. J. Derm. 1970, 83, 248–254. [Google Scholar] [CrossRef]
- Woringer, F. Clear-cell acanthoma. Bull. Soc. Fr. Dermatol. Syphiligr. 1963, 70, 232–233. [Google Scholar]
- Zak, F.G.; Statsinger, A.L. Pale cell acanthoma. Arch. Dermatol. 1966, 93, 674–678. [Google Scholar] [CrossRef]
- Delacretaz, J. Clear Cell Acanthomas. Dermatologica 1964, 129, 147–153. [Google Scholar] [CrossRef]
- Duperrat, B.; Vanbremeersch, F.; David, V.; Mascaro, J.M.; Bedard, A. Giant form of the acanthoma of Degos. Bull. Soc. Fr. Dermatol. Syphiligr. 1966, 73, 884–886. [Google Scholar]
- Tempark, T.; Shwayder, T. Clear cell acanthoma. Clin. Exp. Dermatol. 2012, 37, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, C.J.; Athalye, L. Clear Cell Acanthoma. In StatPearls; Internet; StatPearls Publishing: Treasure Island, Finland, 2020. [Google Scholar]
- Shalin, S.C.; Rinaldi, C.; Horn, T.D. Clear cell acanthoma with changes of eccrine syringofibroadenoma: Reactive change or clue to etiology? J. Cutan. Pathol. 2013, 40, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Grossin, M.; Mazer, J.M.; Auffret, N.; Préaux, J.; Belaïch, S. Rare forms of clear cell acanthoma. Ann. Dermatol. Venereol. 1983, 110, 721–722. [Google Scholar] [PubMed]
- Su, O.; Dizman, D.; Onsun, N.; Ozkaya, D.B.; Tosuner, Z.; Bahali, A.G.; Demirkesen, C. Multiple giant clear cell acanthomas. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kim, N.G.; Oh, C.W. Multiple reddish weeping nodules on the genital area of a girl. Giant clear cell acanthoma (CCA). Clin. Exp. Dermatol. 2010, 35, e67–e69. [Google Scholar] [CrossRef] [PubMed]
- Roytman, M.; Frumkin, A.; Everett, M.A. Giant clear cell acanthoma. J. Am. Acad. Dermatol. 1987, 17, 513–514. [Google Scholar] [CrossRef]
- Langtry, J.A.; Torras, H.; Palou, J.; Lecha, M.; Mascaro, J.M. Giant clear cell acanthoma in an atypical location. J. Am. Acad. Dermatol. 1989, 21, 313–315. [Google Scholar] [CrossRef]
- Arida, M.; English, J.C., 3rd; Mully, T.W. Giant clear-cell acanthoma with keratoacanthoma-like changes: A case report. Dermatol. Online J. 2006, 12, 11. [Google Scholar] [PubMed]
- Murphy, R.; Kesseler, M.E.; Slater, D.N. Giant clear cell acanthoma. Br. J. Dermatol. 2000, 143, 1114–1115. [Google Scholar] [CrossRef] [PubMed]
- Weitzner, S. Clear-cell acanthoma of vermilion mucosa of lower lip. Oral Surg. Oral Med. Oral Pathol. 1974, 37, 911–914. [Google Scholar] [CrossRef]
- Morganroth, P.A.; Jackson, D.; Chaffins, M. Irregular Pigmented Lesion on the Oral Mucosa. JAMA Dermatol. 2014, 150, 563–564. [Google Scholar] [CrossRef]
- Montebugnoli, L.; Felicetti, L.; Cervellati, F.; Foschini, M.P. Glycogenic acanthosis presenting as leukoplakia on the tongue. BMJ Case Rep. 2010, 2010. [Google Scholar] [CrossRef]
- Fyfe, B.S.; Garcia, F.U. Laryngeal Glycogenic acanthosis presenting as leukoplakia. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1029–1030. [Google Scholar] [CrossRef]
- Potenziani, S.; Applebaum, D.; Krishnan, B.; Gutiérrez, C.; Diwan, A.H. Multiple clear cell acanthomas and a sebaceous lymphadenoma presenting in a patient with Cowden syndrome—A case report. J. Cutan. Pathol. 2017, 44, 79–82. [Google Scholar] [CrossRef]
- Turnbull, M.M.; Humeniuk, V.; Stein, B.; Suthers, G.K. Arteriovenous malformations in Cowden syndrome. J. Med. Genet. 2005, 42, e50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toni Alvarez, M.; Perez Alfonzo, R.; Planas-Giron, G. Clear cell acanthoma. Presentation of a case. Med. Cutan. Ibero-Lat.-Am. 1987, 15, 243–246. [Google Scholar] [PubMed]
- Wilde, J.L.; Meffert, J.J.; McCollough, M.L. Polypoid clear cell acanthoma of the scalp. Cutis 2001, 67, 149–151. [Google Scholar] [PubMed]
- Yang, S.G.; Moon, S.H.; Lim, J.G.; Kim, S.D.; Hyun Cho, K. Clear cell acanthoma presenting as polypoid papule combined with melanocytic nevus. Am. J. Dermatopathol. 1999, 21, 63–65. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, J.Y.; Na, J.I.; Byun, H.J.; Cho, K.H. A case of polypoid clear cell acanthoma on the nipple. Ann. Dermatol. 2010, 22, 337–340. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Tatewaki, S.; Takeuchi, M. A case of polypoid clear cell acanthoma on the scrotum. J. Dermatol. 2004, 31, 236–238. [Google Scholar] [CrossRef]
- Inalöz, H.S.; Patel, G.; Knight, A.G. Polypoid clear cell acanthoma: Case report. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 511–512. [Google Scholar] [CrossRef]
- Petzelbauer, P.; Konrad, K. Polypous clear cell acanthoma. Am. J. Dermatopathol. 1990, 12, 393. [Google Scholar] [CrossRef]
- Nijssen, A.; Laeijendecker, R.; Heinhuis, R.J.; Dekker, S.K. Polypoid clear cell acanthoma of unusual size. J. Am. Acad. Dermatol. 2001, 44, 314–316. [Google Scholar] [CrossRef]
- Tanaka, T.; Arai, T.; Ishikawa, T.; Ohnishi, T.; Watanabe, S. Pedunculated clear cell acanthoma. Report of a case with dermoscopic observation. Eur. J. Dermatol. 2010, 20, 132–133. [Google Scholar] [CrossRef] [Green Version]
- Grunwald, M.H.; Rothem, A.; Halevy, S. Atypical clear cell acanthoma. Int. J. Dermatol. 1991, 30, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Zak, F.G.; Girerd, R.J. Clear cell acanthoma (Degos). Hautarzt 1968, 19, 559–561. [Google Scholar] [PubMed]
- Parsons, M.E.; Ratz, J.L. Squamous cell carcinoma in situ arising within clear cell acanthoma. Dermatol. Surg. 1997, 23, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, L.Y.; Kuo, T.T. Malignant Clear Cell Acanthoma: Report of a Rare Case of Clear Cell Acanthoma-Like Tumor with Malignant Features. Am. J. Dermatopathol. 2016, 38, 553–556. [Google Scholar] [CrossRef]
- Shirai, A.; Saeki, H.; Matsuzaki, H.; Ito, K.; Nakagawa, H. Multiple clear cell acanthoma associated with multiple Bowen’s disease. Int. J. Dermatol. 2014, 53, e386–e388. [Google Scholar] [CrossRef]
- Endo, M.; Ohtaki, N.; Takino, C. Clear cell acanthoma of the cheek with Bowenoid transformation. Rinsho Derma 1997, 39, 161–163. [Google Scholar]
- Sánchez Yus, E.; Iglesias Díez, L. Clear cell acanthoma with dendritica cells charged with melanine and fat. Med. Cutan. Ibero-Lat.-Am. 1975, 3, 209–212. [Google Scholar]
- Langer, K.; Wuketich, S.; Konrad, K. Pigmented clear cell acanthoma. Am. J. Dermatopathol. 1994, 16, 134–139. [Google Scholar] [CrossRef]
- Hollmann, K.H.; Civatte, J. Electron microscopic study of clear-cell acanthoma. Ann. Dermatol. Syphiligr. 1968, 95, 139–146. [Google Scholar]
- Fanti, P.A.; Passarini, B.; Varotti, C. Melanocytes in clear cell acanthoma. Am. J. Dermatopathol. 1990, 12, 373–376. [Google Scholar] [CrossRef]
- Bugatti, L.; Filosa, G. Hemosiderotic clear-cell acanthoma: A pigmented mimicker. Indian J. Dermatol. 2011, 56, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Scheinfeld, N. A glistening brown nodule. Pigmented clear cell acanthoma. Arch. Dermatol. 2007, 143, 255–260. [Google Scholar] [CrossRef]
- Saeki, H.; Matsuzaki, H.; Ito, K.; Nobeyama, Y.; Nakagawa, H. Pigmented clear cell acanthoma of the finger simulating pigmented nevus. Int. J. Dermatol. 2014, 53, e579–e581. [Google Scholar] [CrossRef] [PubMed]
- Jacyk, W.K.; Baran, W.; Essop, A. Multiple pigmented clear cell acanthoma in an African patient. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Burg, G.; Würsch, T.; Fäh, J.; Elsner, P. Eruptive hamartomatous clear-cell acanthomas. Dermatology 1994, 189, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, D.; Barduagni, F.; Cerio, R.; Wolter, M. Disseminated eruptive clear cell acanthoma—A case report with review of the literature. Clin. Exp. Dermatol. 1994, 19, 249–253. [Google Scholar] [CrossRef]
- Morillo, V.; Manrique, P.; Zabalza, I.; Artola, J.L. Eruptive clear cell acanthoma. Actas Dermo-Sifiliogr. 2009, 100, 244–246. [Google Scholar] [CrossRef]
- Monari, P.; Farisoglio, C.; Gualdi, G.; Botali, G.; Ungari, M.; Calzavara-Pinton, P. Multiple eruptive clear cell acanthoma. J. Dermatol. Case Rep. 2010, 4, 25–27. [Google Scholar] [CrossRef] [Green Version]
- García-Gavín, J.; González-Vilas, D.; Montero, I.; Rodríguez-Pazos, L.; Pereiro, M.M.; Toribio, J. Disseminated eruptive clear cell acanthoma with spontaneous regression: Further evidence of an inflammatory origin? Am. J. Dermatopathol. 2011, 33, 599–602. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Penneys, N. Cystic clear cell acanthoma. J. Cutan. Pathol. 1995, 22, 188–190. [Google Scholar] [CrossRef]
- Cheng, C.; Lee, Y.; Ko, J.; Yang, C. Subungual clear cell acanthoma. Dermatol. Sin. 2014, 32, 195–196. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Inamoto, N.; Nakamura, K. Two cases of clear cell acanthoma: An immunohistochemical study. J. Cutan. Pathol. 1988, 15, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Zedek, D.C.; Langel, D.J.; White, W.L. Clear-cell acanthoma versus acanthosis: A psoriasiform reaction pattern lacking tricholemmal differentiation. Am. J. Dermatopathol. 2007, 29, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Tiodorovic-Zivkovic, D.; Lallas, A.; Longo, C.; Moscarella, E.; Zalaudek, I.; Argenziano, G. Dermoscopy of clear cell acanthoma. J. Am. Acad. Dermatol. 2015, 72 (Suppl. 1), S47–S49. [Google Scholar] [CrossRef] [PubMed]
- Marco, A.; Buffon, R.B.; Scope, A.; Cota, C.; Bucccini, P.; Berardesca, E.; Pellacani, G.; Marghoob, A.A.; Gill, M. Comparing In Vivo Reflectance Confocal Microscopy, Dermoscopy, and Histology of Clear-Cell Acanthoma. Dermatol. Surg. 2009, 35, 952–959. [Google Scholar]


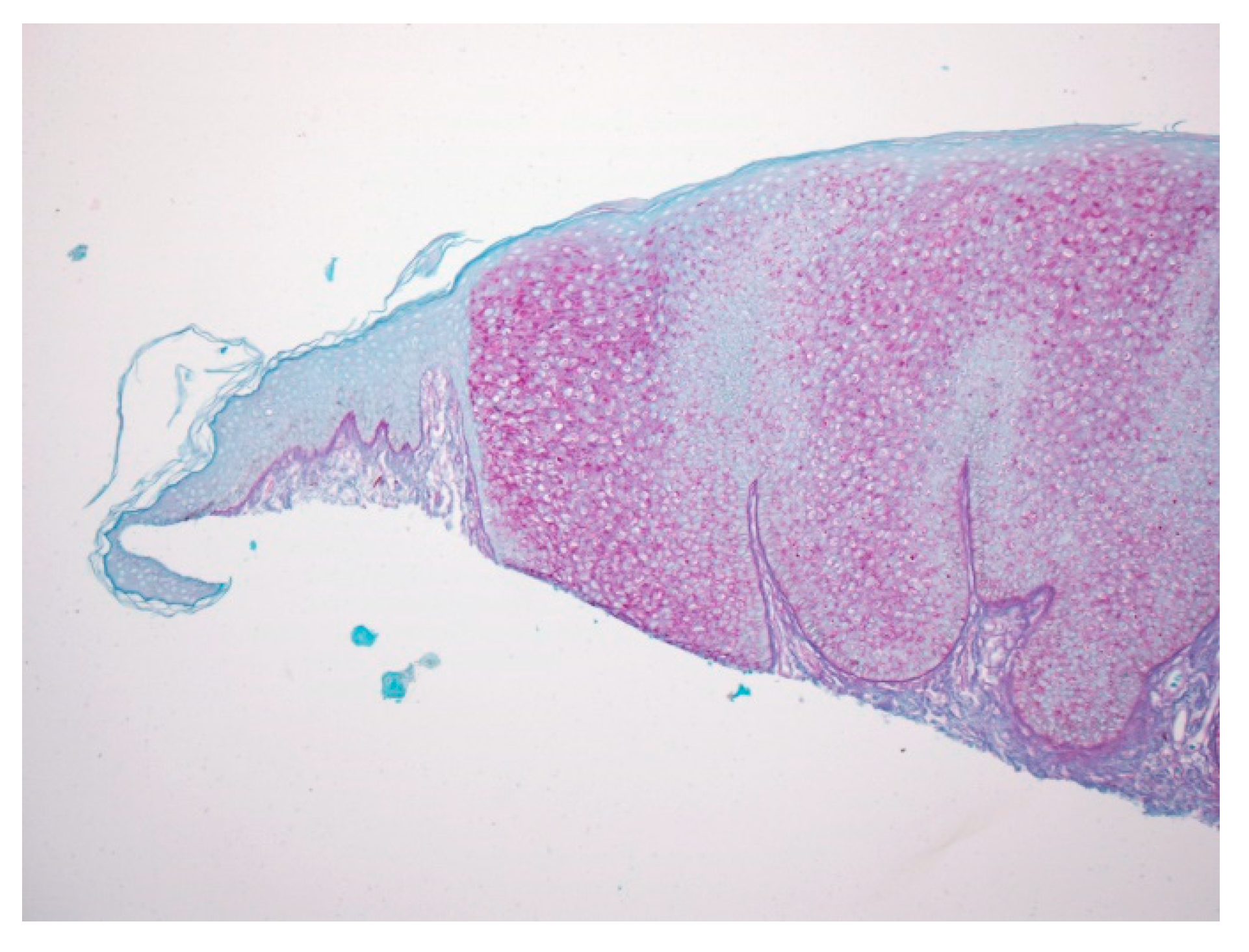
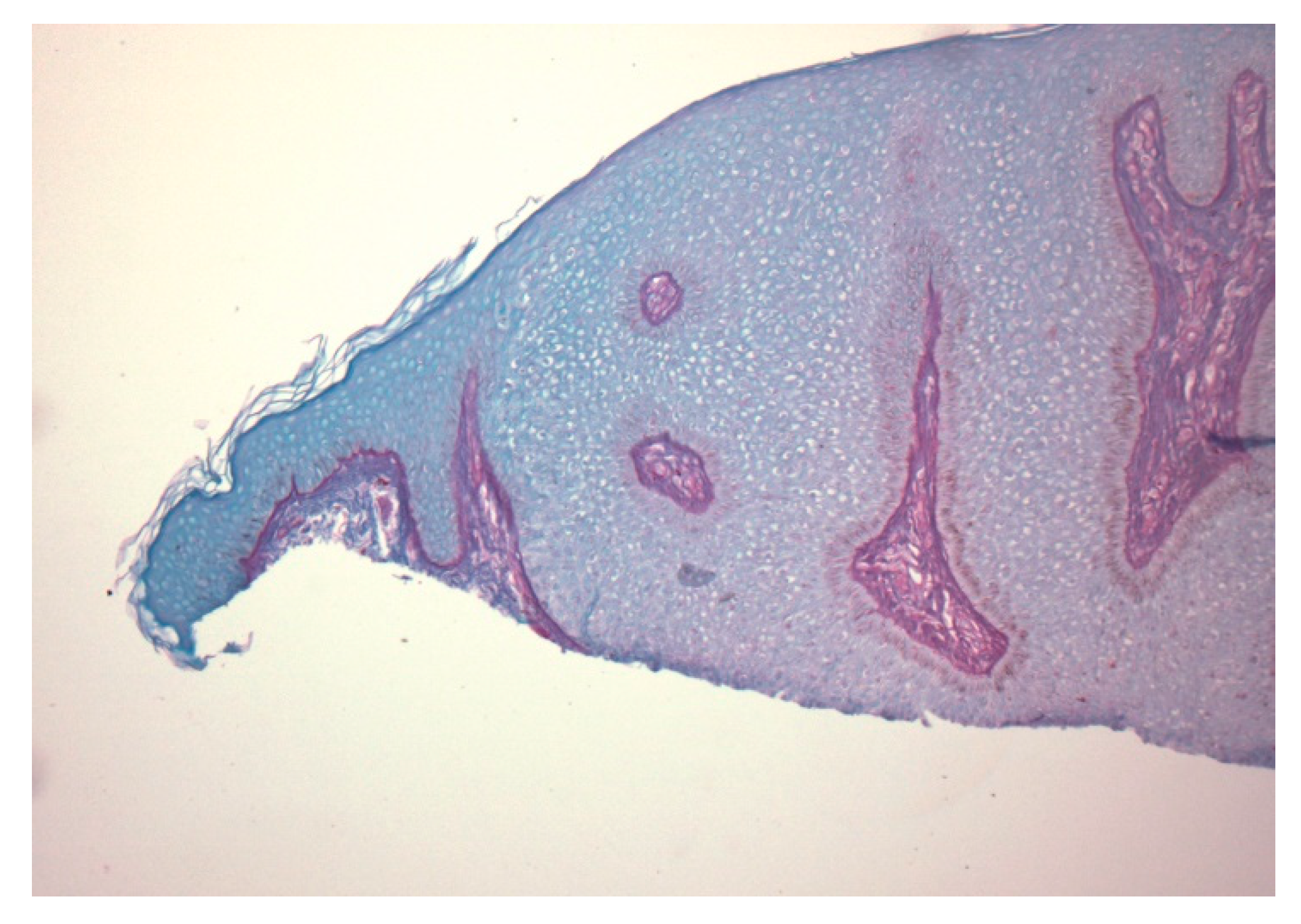

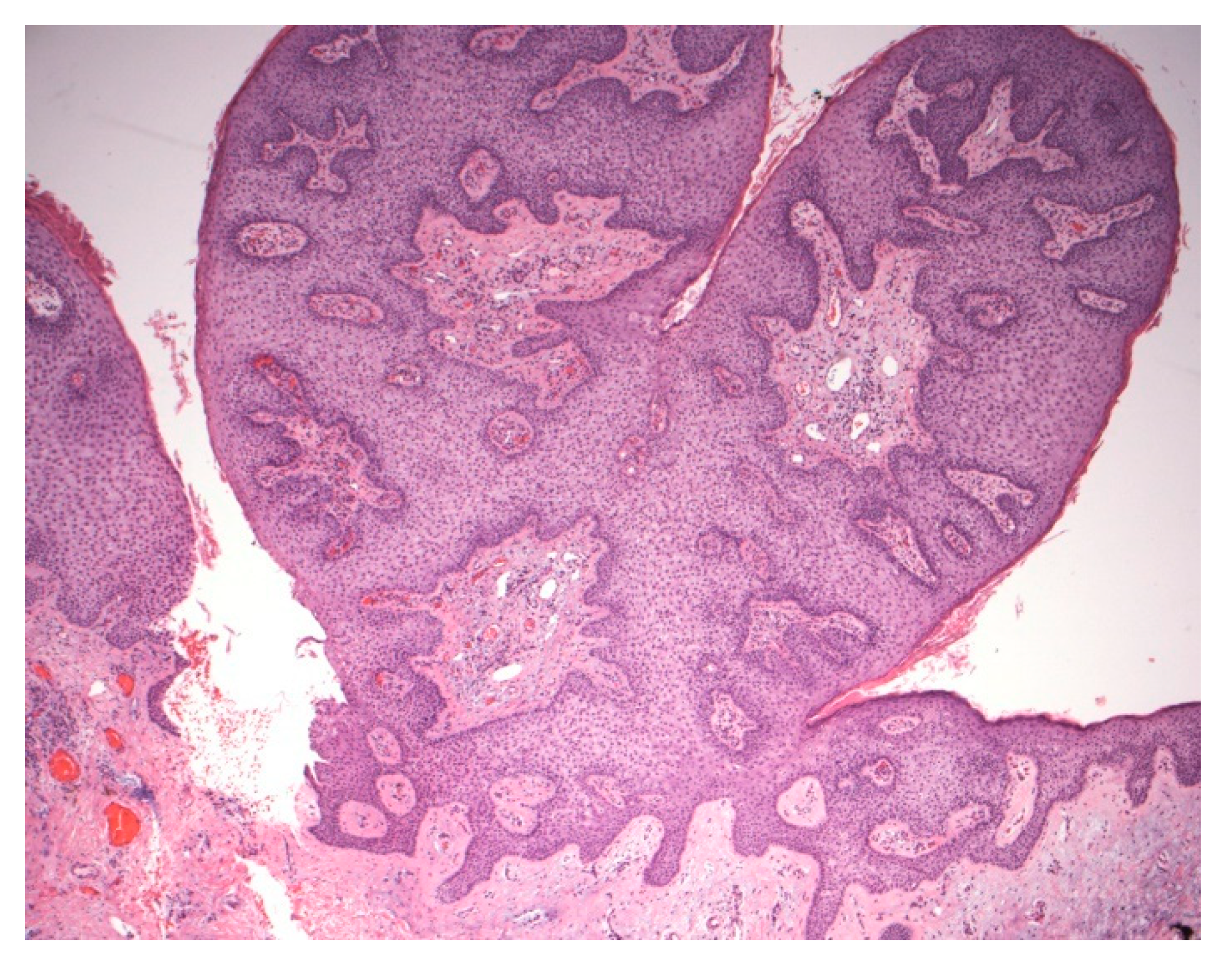



| Type | First Described | Author | Age | Gender | Location of CCA |
|---|---|---|---|---|---|
| Conventional | 1962 | Degos and Civatte | 5–7th decade | No specific inclination | Distal lower extremity |
| Giant | 1966 | Duperrat et al. | Unknown | Unknown | Leg |
| Mucosal | 1974 | Weitzner | 63-year-old | Male | Vermilion mucosa of the lower lip |
| Verrucous | 1987 | Toni Alvarez | 72-year-old | Female | Unknown |
| Polypoid | 1990 | Petzelbauer et al. | 28-year-old | Female | Neck |
| Atypical | 1991 | Grunwald et al. | 7th decade | Male:Female equally | Forehead |
| Pigmented | 1994 | Sanchez and Iglesias | 60-year-old | Male | Abdominal region |
| Eruptive | 1994 | Burg et al. | 38-year-old | Female | Multiple lesions on legs, arms, trunk |
| Cystic | 1995 | Hamaguchi et al. | Middle aged | Male | Suprapubic region |
| Linear | 2012 | Tempark and Shwayder | 4-year-old | Male | Flank |
| Subungual | 2014 | Chun-Yu Cheng et al. | 70-year-old | Male | Fingernail bed |
| Trichilemmal | 2020 | Usmani and Qasim | 74-year-old | Female | Neck |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usmani, A.; Qasim, S. Clear Cell Acanthoma: A Review of Clinical and Histologic Variants. Dermatopathology 2020, 7, 26-37. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology7020005
Usmani A, Qasim S. Clear Cell Acanthoma: A Review of Clinical and Histologic Variants. Dermatopathology. 2020; 7(2):26-37. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology7020005
Chicago/Turabian StyleUsmani, Arif, and Syeda Qasim. 2020. "Clear Cell Acanthoma: A Review of Clinical and Histologic Variants" Dermatopathology 7, no. 2: 26-37. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology7020005






