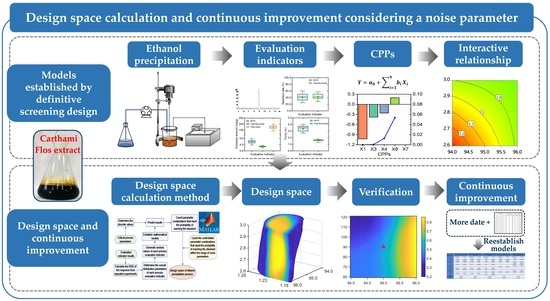
Design Space Calculation and Continuous Improvement Considering a Noise Parameter: A Case Study of Ethanol Precipitation Process Optimization for Carthami Flos Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Ethanol Precipitation
2.3. Experimental Design
2.4. Analytical Method
2.5. Evaluation of Experimental Data
2.6. Calculation of the Design Space
3. Results and Discussion
3.1. Experimental Results of the EPP
3.2. The Identification of CPPs
3.3. Process Modeling of Ethanol Precipitation
3.4. Design Space Development and Verification
3.5. Continuous Improvement Strategy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
Appendix B

| No. | Process Parameters | Extracted Amount (mg/g) | Purity (%) | Retention Rate (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | HSYA | Total Flavonoids | Total Solids | HSYA | Total Flavonoids | Total Solids | HSYA | |
| 1 | 1.23 | 30 | 96 | 3.0 | 160 | 100 | 120 | 10.0 | 48 | 5.69 | 2.85 | 171.42 | 3.32 | 1.66 | 41.15 | 42.18 |
| 2 | 1.23 | 20 | 94 | 2.6 | 120 | 80 | 60 | 2.0 | 24 | 6.55 | 3.00 | 178.39 | 3.67 | 1.68 | 44.43 | 41.70 |
| 3 | 1.28 | 25 | 96 | 2.6 | 160 | 100 | 120 | 2.0 | 24 | 3.81 | 1.92 | 141.84 | 2.68 | 1.35 | 24.76 | 27.88 |
| 4 | 1.18 | 25 | 94 | 3.0 | 120 | 80 | 60 | 10.0 | 48 | 6.77 | 3.09 | 184.24 | 3.67 | 1.68 | 52.44 | 52.76 |
| 5 | 1.28 | 20 | 95 | 3.0 | 120 | 100 | 120 | 10.0 | 24 | 5.12 | 2.64 | 177.52 | 2.88 | 1.48 | 33.40 | 38.33 |
| 6 | 1.18 | 30 | 95 | 2.6 | 160 | 80 | 60 | 2.0 | 48 | 7.48 | 3.35 | 187.38 | 3.99 | 1.79 | 54.49 | 53.73 |
| 7 | 1.28 | 30 | 94 | 2.8 | 160 | 80 | 120 | 10.0 | 48 | 5.32 | 2.54 | 173.66 | 3.06 | 1.46 | 32.79 | 34.96 |
| 8 | 1.18 | 20 | 96 | 2.8 | 120 | 100 | 60 | 2.0 | 24 | 5.75 | 2.69 | 155.68 | 3.69 | 1.73 | 43.69 | 45.00 |
| 9 | 1.28 | 20 | 96 | 2.6 | 140 | 100 | 60 | 10.0 | 48 | 4.85 | 2.64 | 160.59 | 3.02 | 1.64 | 30.58 | 37.05 |
| 10 | 1.18 | 30 | 94 | 3.0 | 140 | 80 | 120 | 2.0 | 24 | 7.15 | 3.08 | 189.33 | 3.78 | 1.63 | 55.43 | 52.47 |
| 11 | 1.28 | 20 | 94 | 3.0 | 120 | 90 | 120 | 2.0 | 48 | 4.87 | 2.24 | 169.11 | 2.88 | 1.33 | 32.10 | 32.99 |
| 12 | 1.18 | 30 | 96 | 2.6 | 160 | 90 | 60 | 10.0 | 24 | 7.11 | 3.16 | 188.08 | 3.78 | 1.68 | 54.77 | 53.56 |
| 13 | 1.28 | 20 | 94 | 2.6 | 160 | 80 | 90 | 10.0 | 24 | 5.17 | 2.68 | 164.54 | 3.14 | 1.63 | 32.63 | 37.75 |
| 14 | 1.18 | 30 | 96 | 3.0 | 120 | 100 | 90 | 2.0 | 48 | 5.84 | 2.49 | 164.97 | 3.54 | 1.51 | 43.06 | 40.46 |
| 15 | 1.28 | 30 | 94 | 2.6 | 120 | 100 | 60 | 6.0 | 48 | 5.98 | 3.17 | 198.66 | 3.01 | 1.60 | 37.17 | 43.96 |
| 16 | 1.18 | 20 | 96 | 3.0 | 160 | 80 | 120 | 6.0 | 24 | 6.54 | 3.05 | 180.19 | 3.63 | 1.69 | 48.34 | 49.57 |
| 17 | 1.28 | 30 | 96 | 2.6 | 120 | 80 | 120 | 2.0 | 36 | 3.98 | 2.10 | 147.89 | 2.69 | 1.42 | 24.47 | 28.81 |
| 18 | 1.18 | 20 | 94 | 3.0 | 160 | 100 | 60 | 10.0 | 36 | 6.12 | 2.64 | 160.55 | 3.81 | 1.65 | 46.04 | 43.74 |
| 19 | 1.28 | 30 | 96 | 3.0 | 120 | 80 | 60 | 10.0 | 24 | 4.92 | 2.78 | 175.38 | 2.80 | 1.59 | 30.33 | 38.24 |
| 20 | 1.18 | 20 | 94 | 2.6 | 160 | 100 | 120 | 2.0 | 48 | 7.57 | 3.27 | 193.73 | 3.91 | 1.69 | 55.71 | 52.96 |
| 21 | 1.28 | 20 | 96 | 3.0 | 160 | 80 | 60 | 2.0 | 48 | 4.00 | 1.96 | 148.82 | 2.69 | 1.31 | 26.14 | 28.48 |
| 22 | 1.18 | 30 | 94 | 2.6 | 120 | 100 | 120 | 10.0 | 24 | 7.77 | 3.38 | 192.25 | 4.04 | 1.76 | 60.25 | 57.75 |
| 23 | 1.28 | 30 | 94 | 3.0 | 160 | 100 | 60 | 2.0 | 24 | 4.72 | 2.55 | 161.21 | 2.93 | 1.58 | 29.71 | 35.83 |
| 24 | 1.18 | 20 | 96 | 2.6 | 120 | 80 | 120 | 10.0 | 48 | 7.16 | 3.25 | 186.24 | 3.85 | 1.74 | 51.97 | 51.84 |
| 25 | 1.23 | 25 | 95 | 2.8 | 140 | 90 | 90 | 6.0 | 36 | 5.95 | 2.83 | 176.00 | 3.38 | 1.61 | 40.99 | 40.01 |
| 26 | 1.23 | 25 | 95 | 2.8 | 140 | 90 | 90 | 6.0 | 36 | 6.44 | 3.17 | 183.62 | 3.51 | 1.73 | 46.28 | 46.69 |
| 27 | 1.23 | 25 | 95 | 2.8 | 140 | 90 | 90 | 6.0 | 36 | 6.07 | 2.82 | 173.67 | 3.50 | 1.62 | 42.16 | 40.09 |
| 28 | 1.23 | 25 | 95 | 2.8 | 140 | 90 | 90 | 6.0 | 36 | 6.08 | 3.05 | 173.48 | 3.50 | 1.76 | 41.37 | 42.59 |
| Parameters | HSYA Extracted Amount | Total Flavonoids Extracted Amount | Total Solids Extracted Amount | ArcSin (Sqrt (HSYA Purity)) | ArcSin (Sqrt (Total Flavonoids Purity)) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | |
| Constant | 6.189 | - | 2.985 | - | 177.6 | - | 0.1879 | - | 0.1298 | - |
| X1 | −1.0238 | <0.0001 | −0.2838 | <0.0001 | −7.4283 | 0.0002 | −0.0126 | <0.0001 | −0.0039 | <0.0001 |
| X3 | −0.3784 | <0.0001 | −0.1261 | 0.0016 | −6.5712 | 0.0006 | −0.0028 | <0.0001 | - | - |
| X4 | −0.2576 | <0.0001 | −0.1157 | 0.0033 | - | - | −0.0022 | <0.0001 | −0.0016 | 0.0128 |
| X7 | - | - | - | - | - | - | - | - | −0.0013 | 0.0346 |
| X8 | 0.1942 | 0.0014 | 0.1358 | 0.0008 | 4.3687 | 0.0129 | 0.0013 | 0.0017 | 0.0018 | 0.0058 |
| X1X4 | - | - | - | - | - | - | 0.0010 | 0.0120 | ||
| X1X7 | - | - | - | - | - | - | - | - | −0.0012 | 0.0572 |
| X1X8 | - | - | - | - | - | - | 0.0014 | 0.0010 | 0.0012 | 0.0599 |
| X3X4 | 0.2068 | 0.0013 | 0.0915 | 0.0235 | 5.1539 | 0.0091 | - | - | - | - |
| X3X8 | 0.3248 | <0.0001 | 0.1747 | 0.0001 | 8.7882 | <0.0001 | - | - | - | - |
| X4X8 | - | - | - | - | - | - | −0.0009 | 0.0354 | - | - |
| X12 | - | - | - | - | 13.2092 | 0.0128 | −0.0046 | <0.0001 | −0.0036 | 0.0098 |
| X32 | −0.3871 | 0.0027 | - | - | −8.2773 | 0.0915 | - | - | - | - |
| X82 | - | - | −0.2348 | 0.0060 | −10.0961 | 0.0409 | - | - | - | - |
| Model p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| R2 | 0.9637 | 0.8803 | 0.8165 | 0.9874 | 0.8102 | |||||
References
- State Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; Volume 1. [Google Scholar]
- Tai, Y.; Shen, J.; Luo, Y.; Qu, H.; Gong, X. Research progress on the ethanol precipitation process of traditional Chinese medicine. Chin. Med. 2020, 15, 84. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, X.; Qu, H. Optimizing the alcohol precipitation of Danshen by response surface methodology. Sep. Purif. Technol. 2013, 48, 977–983. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, B.; Gong, X.; Yu, L.; Qu, H. Application of quality by design to the process development of botanical drug products: A case study. AAPS PharmSciTech 2013, 14, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, X.; Yan, A.; Qu, H. Optimization for the ethanol precipitation process of botanical injection: Indicator selection and factor influences. Sep. Purif. Technol. 2014, 49, 619–626. [Google Scholar] [CrossRef]
- Sun, M.; Yang, J.; Cao, W.; Shao, J.; Wang, G.; Qu, H.; Huang, W.; Gong, X. Critical process parameter identification of manufacturing processes of Astragali Radix extract with a weighted determination coefficient method. Chin. Herb. Med. 2020, 12, 125–132. [Google Scholar] [CrossRef]
- Luo, Y.; Li, W.; Huang, W.; Liu, X.; Song, Y.; Qu, H. Rapid quantification of multi-components in alcohol precipitation liquid of Codonopsis Radix using near infrared spectroscopy (NIRS). J. Zhejiang Univ. SC B 2017, 18, 383–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Qu, H. In-situ monitoring of saccharides removal of alcohol precipitation using near-infrared spectroscopy. J. Innov. Opt. Heal. Sci. 2018, 11, 1850027. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Liu, D.; Du, C.; Lin, L.; Zhu, J.; Huang, X.; Liao, Y.; Wu, Z. Novel NIR modeling design and assignment in process quality control of Honeysuckle flower by QbD. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 242, 118740. [Google Scholar] [CrossRef]
- Pan, J.; Shao, J.; Qu, H.; Gong, X. Ethanol precipitation of Codonopsis Radix concentrate with a membrane dispersion micromixer. J. Clean Prod. 2020, 251, 119633. [Google Scholar] [CrossRef]
- Pan, J.; Tai, Y.; Qu, H.; Gong, X. Optimization of membrane dispersion ethanol precipitation process with a set of temperature control improved equipment. Sci. Rep. 2020, 10, 19010. [Google Scholar] [CrossRef]
- Shao, F.; Yu, M.; Jiang, M.; Shang, Y.; Yang, M.; Liu, R. Effect of Stirring Speed on Fractal Dimension of Ethanol Precipitation of Astragalus Granules and Alcohol Precipitation Effect. J. Chin. Med. Mater. 2019, 42, 612–616. [Google Scholar]
- Meilin, J.; Zhang, X.; Shao, F.; Shang, Y.; Yang, M.; Liu, R.; Mei, H. Effect of ethanol to material ratio on ethanol precipitation and sediment morphology of Shuanghuanglian preparation. Chin. Tradit. Herb Drugs 2020, 51, 4954–4959. [Google Scholar]
- Pan, J.; He, S.; Zheng, J.; Shao, J.; Li, N.; Gong, Y.; Gong, X. The development of an herbal material quality control strategy considering the effects of manufacturing processes. Chin. Med. 2019, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- ICH Guidelines. ICH Q8 (R2) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 24 March 2021).
- Watson, T.; Nosal, R.; Lepore, J.; Montgomery, F. Misunderstanding Design Space: A Robust Drug Product Control Strategy Is the Key to Quality Assurance. J. Pharm. Innov. 2018, 13, 283–285. [Google Scholar] [CrossRef]
- Hersleth, M.; Mevik, B.; Naes, T.; Guinard, J. Effect of contextual factors on liking for wine—Use of robust design methodology. Food Qual. Prefer. 2003, 14, 615–622. [Google Scholar] [CrossRef]
- Zhuang, J.; Liu, S.; Cai, H.; Dai, X.; Chen, Y.; Jin, Z.; Chen, B. Efficacy and safety of Guhong injection for treating coronary microvascular disease: Study protocol for a randomized controlled trial. Trials 2020, 21, 75. [Google Scholar]
- Sun, Z.; Jin, H.; Zhou, H.; Yu, L.; Wan, H.; He, Y. Guhong Injection promotes fracture healing by activating Wnt/beta-catenin signaling pathway in vivo and in vitro. Biomed. Pharmacother. 2019, 120, 109436. [Google Scholar] [CrossRef]
- Ai, J.; Wan, H.; Shu, M.; Zhou, H.; Zhao, T.; Fu, W.; He, Y. Guhong injection protects against focal cerebral ischemia–reperfusion injury via anti-inflammatory effects in rats. Arch. Pharm. Res. 2017, 40, 610–622. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Chen, L.; Xu, M.; Wu, J.; Zhang, P.; Nel, D.; Sun, B. Protective effect of hydroxysafflor yellow A on dopaminergic neurons against 6-hydroxydopamine, activating anti-apoptotic and anti-neuroinflammatory pathways. Pharm. Biol. 2020, 58, 686–694. [Google Scholar] [CrossRef]
- Bai, X.; Wang, W.; Fu, R.; Yue, S.; Gao, H.; Chen, Y.; Tang, Y. Therapeutic Potential of Hydroxysafflor Yellow A on Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2020, 11, 01265. [Google Scholar] [CrossRef]
- Han, D.; Wei, J.; Zhang, R.; Ma, W.; Shen, C.; Feng, Y.; Xia, N.; Xu, D.; Cai, D.; Li, Y.; et al. Hydroxysafflor yellow A alleviates myocardial ischemia/reperfusion in hyperlipidemic animals through the suppression of TLR4 signaling. Sci. Rep. 2016, 6, 35319. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Du, Z.; Li, Y.; Gao, P.; Song, J.; Lu, Y.; Tu, P.; Jiang, Y.; Guo, X. The synergistic mechanism of total saponins and flavonoids in Notoginseng-Safflower pair against myocardial ischemia uncovered by an integrated metabolomics strategy. Biomed. Pharmacother. 2020, 130, 110574. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Wang, S.; Li, Y.; Qu, H. Separation characteristics of ethanol precipitation for the purification of the water extract of medicinal plants. Sep. Purif. Technol. 2013, 107, 273–280. [Google Scholar] [CrossRef]
- Gong, X.; Chen, H.; Chen, T.; Qu, H. Unit operation optimization for the manufacturing of botanical injections using a design space approach: A case study of water precipitation. PLoS ONE 2014, 9, e104493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Li, W.; Qu, H.; Tian, G.; Wei, Y. Application of definitive screening design to quantify the effects of process parameters on key granule characteristics and optimize operating parameters in pulsed-spray fluid-bed granulation. Particuology 2019, 43, 56–65. [Google Scholar] [CrossRef]
- WS-10001-(HD-1506)-2004-2012; Guhong Injection; National Food and Drug Administration National Drug Standards: Shanxi, China, 2012.
- Gong, X.; Li, Y.; Chen, H.; Qu, H. Design Space Development for the Extraction Process of Danhong Injection Using a Monte Carlo Simulation Method. PLoS ONE 2015, 10, e0128236. [Google Scholar] [CrossRef] [Green Version]
- Dahmash, E.Z.; Al-Khattawi, A.; Iyire, A.; Al-Yami, H.; Dennison, T.J.; Mohammed, A.R. Quality by Design (QbD) based process optimisation to develop functionalised particles with modified release properties using novel dry particle coating technique. PLoS ONE 2018, 13, e0206651. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, J.-E. Application of Open Source Based DoE R Program for the Development of QbD. Yakhak Hoeji 2019, 63, 274–281. [Google Scholar] [CrossRef]
- Shao, J.; Cao, W.; Qu, H.; Pan, J.; Gong, X. A novel quality by design approach for developing an HPLC method to analyze herbal extracts: A case study of sugar content analysis. PLoS ONE 2018, 13, e0198515. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Fu, H.; Zhang, S.; Qu, H. Combining convolutional neural networks and in-line near-infrared spectroscopy for real-time monitoring of the chromatographic elution process in commercial production of notoginseng total saponins. J. Sep. Sci. 2020, 43, 663–670. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, S.; Fu, H.; Qu, H. Combining convolutional neural networks and on-line Raman spectroscopy for monitoring the Cornu Caprae Hircus hydrolysis process. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 226, 117589. [Google Scholar] [CrossRef]










| Factors | Symbols | Coded Values | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Density of the concentrated extract (g/cm3) | X1 | 1.18 | 1.23 | 1.28 |
| Temperature of the concentrated extract (°C) | X2 | 20 | 25 | 30 |
| Ethanol concentration (%) | X3 | 94 | 95 | 96 |
| ECR (v/v) | X4 | 2.6 | 2.8 | 3.0 |
| Stirring speed (rpm) | X5 | 120 | 140 | 160 |
| Time of ethanol addition (min) | X6 | 80 | 90 | 100 |
| Stirring time after ethanol addition (min) | X7 | 60 | 90 | 120 |
| Refrigeration temperature (°C) | X8 | 2.0 | 6.0 | 10.0 |
| Refrigeration time (h) | X9 | 24 | 36 | 48 |
| CPPs | Inside the Design Space | Outside the Design Space | ||||
|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V4 | V5 | V6 | |
| X1 (g/cm3) | 1.23 | 1.23 | 1.23 | 1.19 | 1.19 | 1.19 |
| X3 (%) | 94.8 | 94.8 | 94.8 | 95.0 | 95.0 | 95.0 |
| X4 (v/v) | 2.96 | 2.96 | 2.96 | 2.96 | 2.96 | 2.96 |
| X7 (min) | 120 | 120 | 120 | 90 | 90 | 90 |
| X8 (°C) | 2.0 | 6.0 | 10.0 | 2.0 | 6.0 | 10.0 |
| Calculated probability(%) | 93.1 | 94.0 | 96.0 | 97.0 | 52.0 | 76.0 |
| Evaluation Indicators | Value Types | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|---|
| HSYA extracted amount | PV (mg/g) | 5.88 | 6.01 | 6.14 | 6.61 | 6.8 | 7 |
| EV (mg/g) | 5.94 | 6.07 | 6.39 | 6.51 | 6.98 | 6.94 | |
| ARD (%) | 0.98 | 0.92 | 3.95 | 1.5 | 2.56 | 0.86 | |
| Total flavonoids extracted amount | PV (mg/g) | 2.57 | 2.9 | 2.77 | 2.75 | 3.12 | 3.02 |
| EV (mg/g) | 2.68 | 2.7 | 2.85 | 2.94 | 3.02 | 3.43 | |
| ARD (%) | 4.11 | 7.47 | 2.76 | 6.67 | 3.23 | 12.02 | |
| Total solids extracted amount | PV (mg/g) | 163.3 | 178.4 | 168.9 | 173.1 | 189.1 | 181.8 |
| EV (mg/g) | 160.8 | 164.2 | 169.1 | 170.8 | 183.6 | 189.7 | |
| ARD (%) | 1.57 | 8.65 | 0.13 | 1.37 | 3.01 | 4.17 | |
| HSYA purity | PV (mg/g) | 3.42 | 3.44 | 3.47 | 3.68 | 3.64 | 3.72 |
| EV (mg/g) | 3.69 | 3.69 | 3.78 | 3.81 | 3.8 | 3.66 | |
| ARD (%) | 7.34 | 6.77 | 8.17 | 3.42 | 4.26 | 1.75 | |
| Total flavonoids purity | PV (mg/g) | 1.57 | 1.61 | 1.66 | 1.65 | 1.67 | 1.69 |
| EV (mg/g) | 1.67 | 1.64 | 1.68 | 1.72 | 1.65 | 1.81 | |
| ARD (%) | 5.92 | 1.96 | 1.66 | 4.53 | 1.24 | 6.79 |
| Parameters | HSYA Extracted Amount | Total Flavonoids Extracted Amount | Total Solids Extracted Amount | ArcSin (Sqrt (HSYA Purity)) | ArcSin (Sqrt (Total Flavonoids Purity)) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | Coefficients | p Value | |
| Constant | 6.22 | - | 2.95 | - | 175.18 | - | 0.19 | - | 0.131 | - |
| X1 | −1.02 | <0.0001 | −0.2893 | <0.0001 | −7.36 | <0.0001 | −0.0126 | <0.0001 | −0.004 | <0.0001 |
| X3 | −0.3808 | <0.0001 | −0.1232 | 0.0010 | −6.49 | 0.0001 | −0.003 | <0.0001 | - | - |
| X4 | −0.2508 | <0.0001 | −0.1221 | 0.0007 | −2.96 | 0.0423 | −0.0017 | 0.0094 | −0.0015 | 0.0115 |
| X7 | - | - | - | - | - | - | - | - | −0.0012 | 0.0349 |
| X8 | 0.2031 | <0.0001 | 0.143 | <0.0001 | 4.87 | 0.0011 | - | - | 0.0017 | 0.0037 |
| X1X7 | - | - | - | - | - | - | - | - | −0.0016 | 0.0130 |
| X1X8 | - | - | - | - | - | - | 0.0013 | 0.0502 | - | - |
| X3X4 | 0.2045 | 0.0001 | 0.1179 | 0.0024 | 5.44 | 0.0020 | - | - | - | - |
| X3X8 | 0.3228 | <0.0001 | 0.1778 | <0.0001 | 8.82 | <0.0001 | - | - | - | - |
| X7X8 | - | - | - | - | - | - | - | - | 0.0024 | 0.0937 |
| X12 | - | - | - | - | 13.47 | 0.0015 | −0.0067 | <0.0001 | −0.0027 | 0.0494 |
| X32 | −0.4161 | 0.0001 | −0.1978 | 0.0022 | −7.9 | 0.0302 | - | - | - | - |
| X82 | - | - | - | - | −8.16 | 0.0189 | - | - | - | - |
| Model p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| R2 | 0.9636 | 0.8707 | 0.8306 | 0.9458 | 0.7888 | |||||
| Value Types | Extracted Amount (mg/g) | Purity (%) | |||
|---|---|---|---|---|---|
| HSYA | Total Flavonoids | Total Solids | HSYA | Total Flavonoids | |
| PV (mg/g) | 6.52 | 3.08 | 170.1 | 3.75 | 1.75 |
| EV (mg/g) | 6.63 | 2.68 | 173.3 | 3.82 | 1.54 |
| ARD (%) | 1.66 | 14.93 | 1.85 | 1.83 | 13.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, Y.; Qu, H.; Gong, X. Design Space Calculation and Continuous Improvement Considering a Noise Parameter: A Case Study of Ethanol Precipitation Process Optimization for Carthami Flos Extract. Separations 2021, 8, 74. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8060074
Tai Y, Qu H, Gong X. Design Space Calculation and Continuous Improvement Considering a Noise Parameter: A Case Study of Ethanol Precipitation Process Optimization for Carthami Flos Extract. Separations. 2021; 8(6):74. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8060074
Chicago/Turabian StyleTai, Yanni, Haibin Qu, and Xingchu Gong. 2021. "Design Space Calculation and Continuous Improvement Considering a Noise Parameter: A Case Study of Ethanol Precipitation Process Optimization for Carthami Flos Extract" Separations 8, no. 6: 74. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8060074