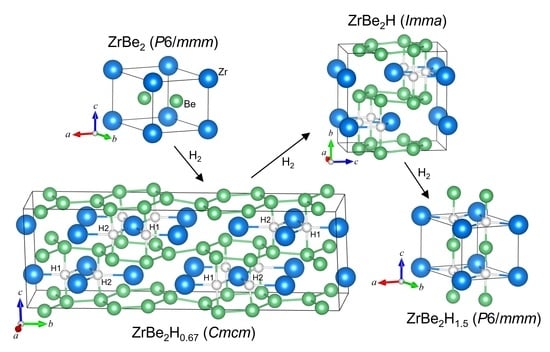
3.1. Neutron Powder Diffraction
NPD patterns were collected for the ZrBe
2D
1.5, ZrBe
2H
1.5, ZrBe
2H
1.2, ZrBe
2H
0.9, and ZrBe
2H
0.6 samples at various temperatures. Select patterns and accompanying structure refinements are shown in the
Supplementary Materials.
Table 1 lists the structural phases found at room temperature. All samples contained additional small Bragg features due to 2% to 3% hexagonal ZrBe
5 impurity phase [
33], consistent with previous NPD measurements [
16].
The ZrBe
2D
1.5 sample was measured at 4 K and 300 K with better statistics and resolution than in [
16] in an attempt to shed more light on the low-temperature ordered structure (see
Figures S1 and S2). At 300 K, besides the fundamental Bragg peaks from the known
P6/
mmm structure (in
Figure 1) and small peaks from the ZrBe
5 impurity, there are also additional trace Bragg peaks that do not belong to any reported Zr-Be solid solution phases and cannot be indexed to a single phase. At 4 K, besides these trace peaks already present at 300 K, there are numerous additional weak satellite peaks, some of which were previously identified in [
16] as due to some type of ordering that may involve laterally modulated
c-axis displacements of the D atoms. As both sets of peaks at 300 K and 4 K are not compatible with the fundamental Bragg peaks of hexagonal ZrBe
2D
1.5, i.e., the
d-spacing associated with these peaks cannot be expressed by the lattice parameters of the basic
P6/
mmm ZrBe
2D
1.5 structure with the corresponding integer-
hkl relations, they cannot be indexed together with the average structure into a conventional single phase. Hence, the complicating pattern of these extra small Bragg peaks precludes any better understanding of the exact nature of the low-temperature structural ordering that occurs for ZrBe
2D
1.5. We can only speculate that, although the trace peaks present at both 300 K and 4 K are of unknown origin and may well reflect minor impurities, the second set of peaks appearing at 4 K due to the low-temperature ordering probably signal an
incommensurately modulated structure induced by contributions from both D displacements and vacancy ordering. Structural parameters at 4 K and 300 K are listed in
Table S1. At 300 K, the refined lattice parameters based on a disordered hexagonal structure (while ignoring the first set of extra trace peaks) are
a = 3.716718 (3) Å,
c = 3.474097 (3) Å, and
V = 41.562 (1) Å
3.
NPD patterns for the ZrBe
2H
1.5 sample at 15 K, 300 K, 400 K, and 500 K indicated the same
P6/
mmm structure as reported for ZrBe
2D
1.5 (see
Figures S3 and S4). Interestingly, there were no extra satellite peaks appearing in the 15 K pattern to mark the occurrence of long-range ordering. If present, they were too diffusive to be recognizable, any delineation being further hindered by the higher incoherent scattering background associated with H compared to D. It is always possible that any low-temperature ordering in ZrBe
2H
1.5 is inherently of much shorter range compared to that for ZrBe
2D
1.5, leading to excessive satellite peak broadening. Structural parameters are listed in
Tables S2 and S3. At 300 K,
a = 3.71971 (3) Å,
c = 3.47680 (4) Å, and
V = 41.661 (1) Å
3, values only slightly higher than for ZrBe
2D
1.5.
In contrast to the ZrBe
2H
1.5 sample, structural refinements for the three ZrBe
2H
x samples with relatively less H content indicated the presence at room temperature of two new structurally unique, orthorhombic, H-sublattice-ordered ZrBe
2H
x phases coinciding with respective nominal
x values of 1 and 0.67. Although the model fits for the ZrBe
2H phase yielded
x values close to 1, the model fits for the ZrBe
2H
0.67 phase yielded
x values more noticeably less than the ideal 0.67, suggesting that this ordered structure may prefer to be more H-deficient due to the presence of partial H-site occupations. Nonetheless, for simplicity in the ensuing discussions, the two new structural compounds will be referred to as ZrBe
2H and ZrBe
2H
0.67. ZrBe
2H is the only compound phase found in the ZrBe
2H
0.9 sample, whereas both compound phases are found in the ZrBe
2H
0.6 sample with ZrBe
2H
0.67 being the roughly 80 % majority phase. (The minor presence of ZrBe
2H in the ZrBe
2H
0.6 sample is likely due to incomplete homogenization of H among ZrBe
2H
x crystallites during the formation of the ZrBe
2H
0.6 sample from the ZrBe
2H
0.9 sample.) The NPD data for the ZrBe
2H
0.9 and ZrBe
2H
0.6 samples (
Figures S8–S11) were the respective bases for structural determinations of these new ZrBe
2H and ZrBe
2H
0.67 phases. The NPD data for the ZrBe
2H
1.2 sample (e.g.,
Figures S5–S7), which was found to contain both ZrBe
2H and ZrBe
2H
~1.3–1.4 (i.e
., more H-deficient ZrBe
2H
1.5) compound phases in a roughly 2:1 ratio, was also used to follow more extensively the temperature behavior of the ZrBe
2H phase.
In both ZrBe
2H and ZrBe
2H
0.67 model structures, the H atoms are located at the center of the trigonal bipyramidal BeZr
3Be sites. Models incorporating additional positional disorder due to small H displacements from the Zr plane to the two off-center tetrahedral Zr
3Be sites above and below did not show significant improvements compared to that for bipyramidal occupation regarding the goodness-of-fit R-factors, H displacement parameters, etc., so they were not considered further. This is in line with the previous DFT results [
10], which suggested that the tendency for favorable off-center H displacements in ZrBe
2H
x disappears for
x ≤ 1.
Figure 2 shows the ordered ZrBe
2H structure. It possesses orthorhombic
Imma symmetry. It should be noted that, for comparisons with the hexagonal
P6/
mmm structure in
Figure 1, the orthorhombic
b-axis of ZrBe
2H in
Figure 2 corresponds to the hexagonal
c-axis in
Figure 1.
Tables S4–S7 summarize the ZrBe
2H structural parameters. At 295 K,
a = 3.8064 (2) Å,
b = 6.55939 (9) Å,
c = 6.5947 (3) Å, and
V = 164.655 (4) Å
3. Model refinements indicate that H atoms in orthorhombic ZrBe
2H occupy specific trigonal Zr
3 (4
e) sites with near unity occupation (0.98 (1)) in the Zr
ac-planes.
Figure 2b shows the zigzag chains of H atoms in the Zr planes that form along the
a-axis, separated by zigzag chains of vacancies. In neighboring Zr
ac-planes, this arrangement of chains is nominally shifted in both the
a-direction and
c-direction by one-half of a unit cell. The presence of H in a particular BeZr
3Be trigonal bipyramid causes a contraction of the Zr
3 triad (due to Zr-H attraction) and an expansion of the Be-Be distance (due to Be-H repulsion). This results in comparatively expanded vacant Zr
3 triads (yielding slight in-plane deviations from the ideal trigonal Zr planar network) and a rippling of the Be honeycomb
ac-layers. At 295 K, the filled and empty BeZr
3Be trigonal bipyramids display respective Be-Be separations of 3.496 (2) Å and 3.063 (2) Å, the latter separation presumably too small to accommodate an H atom. The H positions possess Be-H separations of 1.7481 (8) Å and Zr-H separations of 2.13 (2) Å and 2.145 (8) Å.
NPD patterns for ZrBe
2H at different temperatures between 10 K and 480 K indicate that a transition from the orthorhombic ordered
Imma phase to the hexagonal disordered
P6/
mmm phase with half-filled H sites (depicted in
Figure 1) occurs near 460 K. This transition is reflected by the observed temperature progression of the (011) Bragg peak intensity for orthorhombic ZrBe
2H in
Figure 3, a reflection that is forbidden for
P6/
mmm symmetry.
The more extensive temperature-dependent NPD data collected for the ZrBe
2H
1.2 sample suggested that the observed decay of the (011) Bragg peak intensity with increasing temperature for the ZrBe
2H order-disorder phase transition is not due to a spillover of H into other crystallographically distinct, vacant sites, such as marked in
Figure 2, but rather mainly by an increasing displacement parameter. Indeed, according to the Fourier difference map, only the one H 4
e position was evident in the orthorhombic ZrBe
2H structure at all temperatures measured below the 460 K phase-transition temperature. Moreover, for all ZrBe
2H
1.2 sample data below 460 K, refinement of the 4
e site occupancy resulted in values exceeding unity, so its value was fixed at 1 in all subsequent model refinements. (See
Figures S5 and S6 and Table S4).
Figure 4 shows the ordered ZrBe
2H
0.67 structure. It possesses orthorhombic
Cmcm symmetry.
Table S8 summarizes the ZrBe
2H
0.67 structural parameters. At 295 K,
a = 3.8161 (2) Å,
b = 19.8247 (11) Å,
c = 6.4997 (1) Å, and
V = 491.724 (14) Å
3.
Model refinements associated with the ZrBe
2H
0.6 sample at 45 K (see
Figure S9) indicate that H atoms in the orthorhombic ZrBe
2H
0.67 phase occupy two crystallographically distinct trigonal Zr
3 (4
c) sites (H1 and H2), both with the same immediate surroundings, being at the center of BeZr
3Be trigonal bipyramids.
Figure 4b shows that these H1 and H2 atoms form double chains in the Zr planes along the
a-axis, each double chain separated by four parallel chains of trigonal Zr
3 vacancies. In neighboring Zr
ab-planes, H1 and H2 designations are swapped and the general arrangement of double chains is nominally shifted in the
a-direction by one-half of a unit cell and in the
b-direction by one-sixth of a unit cell.
As for orthorhombic ZrBe
2H, the presence of an H atom in a particular BeZr
3Be trigonal bipyramid of ZrBe
2H
0.67 causes a contraction of the Zr
3 triad and an expansion of the Be-Be distance, again resulting in comparatively expanded vacant Zr
3 triads and a rippling of the Be honeycomb
ab-layers (see
Figure 4). At 295 K, the H1-filled and H2-filled BeZr
3Be trigonal bipyramids display respective Be-Be separations of 3.571 (8) Å and 3.299 (7) Å. The four types of empty BeZr
3Be trigonal bipyramids display varying Be-Be separations of 2.874 (8) Å, 2.929 (8) Å, 3.200 (7) Å, and 3.626 (8) Å, the relatively large latter separation associated with the bipyramids containing the vacant H3 positions marked by a red × in
Figure 4b,d. The H1 positions possess Be-H1 separations of 1.786 (4) Å and Zr-H1 separations of 2.05 (2) Å and 2.202 (9) Å. The H2 positions possess Be-H2 separations of 1.650 (4) Å and Zr-H2 separations of 2.14 (4) Å and 2.19 (2) Å.
The differences in H ordering within the Zr planes between orthorhombic ZrBe
2H
0.67 and ZrBe
2H shown in
Figure 2d and
Figure 4b are noteworthy. In ZrBe
2H
0.67, H atoms tend to localize in close pairs (with 2.17 (4) Å H-H separations at 295 K) situated in the double Zr
3 centers of a chain of toe-to-toe Zr triangle pairs with one shared edge, whereas in ZrBe
2H, all H atoms localize in zigzag chains with two close H neighbors (with 2.22 (2) Å H-H separations at 295 K), each H atom situated in the Zr
3 centers of a zigzag chain of shoulder-to-shoulder Zr triangles with two shared edges. We note that the close H-H distances in all the ZrBe
2H
x compounds studied are comparable to the ~2.1 Å minimum value necessary for hydride stability [
34], with the lowest value of 2.08 (4) Å determined for ZrBe
2H
0.67 at 45 K. Unlike the DFT results for vacancy-ordered ZrBe
2H
1.5 structures where maximum vacancy separation seems to be favored [
14], the H ordering in the more H-dilute ZrBe
2H and ZrBe
2H
0.67 suggests that there is some energetic advantage to specific clustering patterns for both H and vacancies.
The 45 K and 295 K patterns for ZrBe
2H
0.67 (
Figures S9 and S10) look similar and could be fit using the same model containing two H positions, H1 and H2. According to the refinements, the H1 site is close to fully occupied whereas the H2 site is partially occupied (e.g., ≈82 (4)% at 45 K). We note that the 295 K pattern could also be fit equally well using an alternative model with a small amount of H partially occupying a third 4
c position, H3, already mentioned above (which is located at the centers of the BeZr
3Be trigonal bipyramids neighboring the H1 positions; see
Figure 4b,d and
Table S8), and thus all three sites, H1, H2, and H3, have partial occupancies of around 0.79 (6), 0.75 (7), and 0.26 (7), respectively. For the 45 K pattern, the three-site model diverged during refinement and thus was not representative at this temperature. Although only tentative based solely on these two NPD patterns, the refinement results suggest that the two-site model at 45 K may gradually transform towards a three-site model as the temperature is increased, with some minor amount of H entering the neighboring empty trigonal bipyramid site already by 295 K. Of course, this would be a different thermal behavior than the apparent lack of temperature-induced H site spillover observed for orthorhombic ZrBe
2H. We note that this particular spillover site does make steric sense since the associated BeZr
3Be trigonal bipyramid has an unusually elongated Be-Be separation (3.626 (8) Å at 295 K) that is even larger than those respective Be-Be separations (3.571 (8) Å and 3.299 (7) Å) corresponding to the filled H1 and H2 sites. Indeed, this differs dramatically from the prohibitively short 3.063 (2) Å Be-Be separations for the empty BeZr
3Be trigonal bipyramids in orthorhombic ZrBe
2H.
In contrast to the 45 K and 295 K patterns, the 500 K pattern for ZrBe
2H
0.67 (
Figure S11) indicates a change from the ordered orthorhombic structure to the same disordered hexagonal
P6/
mmm structure displayed in
Figure 1 by disordered ZrBe
2H
1.5 and ZrBe
2H, with H partially occupying, at random, one-third of all BeZr
3Be trigonal bipyramids. By following the intensity of the characteristic (021) Bragg reflection associated with
Cmcm symmetry as a function of temperature up to 510 K (see
Figure 3), we ascertained that the phase transition from orthorhombic
Cmcm to hexagonal
P6/
mmm occurs at ≈ 490 K, which is roughly 30 K and 290 K higher than the respective order-disorder transition temperatures for ZrBe
2H and ZrBe
2H
1.5. Again, as we have full NPD patterns for the ZrBe
2H
0.6 sample at only two temperatures, 45 K and 295 K, we cannot completely discount H site spillover as at least partially responsible (besides increasing H displacement parameters) for the temperature-dependent decay of the (021) Bragg peak intensity for orthorhombic ZrBe
2H
0.67 in
Figure 3.
As mentioned earlier, the ZrBe
2H
1.2 sample was found to be a mixture of ZrBe
2H and ZrBe
2H
~1.3–1.4 phases at all temperatures. Below 460 K, the ZrBe
2H phase is ordered orthorhombic and the ZrBe
2H
~1.3–1.4 phase is disordered hexagonal. Above 460 K, orthorhombic ZrBe
2H transforms to the
P6/
mmm structure (i.e., the same disordered model structure as hexagonal ZrBe
2H
~1.3–1.4 but with a different H content and thus different lattice parameters). The H content of the ZrBe
2H
~1.3–1.4 phase is based on the refined values for H occupancy and marks the lower limit of H content for the nominally hexagonal ZrBe
2H
1.5 phase. A comparison of NPD data at various temperatures (e.g., see
Figures S5–S7) suggest that the lower
x limit at 300 K and below is ~1.4 and may drift down to ~1.3 by 460 K. Yet, this observation is tentative since, at high temperatures, H site occupancies are highly correlated to their atomic displacement parameters, precluding their accurate determination. The 460 K value is estimated only after reasonably fixing the displacement parameters. It is not clear what the upper
x limit is for this phase, although the previous NMR studies of ZrBe
2D
x indicated an
x value as high as 1.56 is possible [
17]. Nonetheless, from the current and past results, it seems clear that the hexagonal ZrBe
2H
1.5 phase can exist for a range of H content (from
x ≈ 1.3 to >1.5).
As the 300 K volume/f.u. for the ZrBe
2H phase in the ZrBe
2H
1.2 sample is found to be slightly larger by ~0.3% than that at similar temperature for the ZrBe
2H phase in the ZrBe
2H
0.9 sample (see
Tables S4 and S7), this suggests that there is a real, albeit slight, difference in H content for the nominal ZrBe
2H phase in the two samples. This is consistent with the occurrence of refined H 4
e site occupancies always >1 for the ZrBe
2H phase in the ZrBe
2H
1.2 sample (necessitating its fixed value of 1) compared to a refined occupancy value of 0.98(1) for that in the ZrBe
2H
0.9 sample. Hence, this signals that there is likely some limited range of H content possible (
x ≈ 0.98 to 1) for a stabilized ZrBe
2H phase.
A plot of the hexagonal
P6/
mmm lattice constants for ZrBe
2H
x near or at 500 K as functions of increasing
x in
Figure 5 indicates a decreasing
a lattice constant concomitant with a more steeply increasing
c lattice constant. The net result is an increasing unit cell volume
V with increasing H content. For dilute H contents (i.e.,
x < 0.67), the changes in
a and
c and the increase in
V are minimal but all become more dramatic as the H content increases above
x = 0.67. This corresponds well to the observation by Westlake [
13] that the H
2 absorption isotherm data at 588 K [
11] for
x ≤ 0.5 closely follow Sieverts’ Law [
35] (i.e., the solubility of a diatomic gas in a metal is proportional to the square root of the partial pressure of the gas in thermodynamic equilibrium). Indeed, in this more dilute solid-solution region, the overall ZrBe
2 framework dimensions are little perturbed by the inclusion of H atoms, and
x is found to be proportional to (
PH2)
½. In contrast, at higher
x values, the ZrBe
2 framework dimensions become more markedly affected by the more numerous H atoms, leading to a significant positive deviation from Sieverts’ Law. As for the orthorhombic ordered ZrBe
2H
0.67 and ZrBe
2H phases, within this hexagonal solid-solution phase, the decreasing
a lattice constant reflects a basal-plane contraction due to the attractive Zr-H interactions, whereas the increasing
c lattice constant reflects the repulsive Be-H interactions.
Figure 6 conveys the lattice expansion behavior of the three compounds ZrBe
2H
0.67, ZrBe
2H, and ZrBe
2H
1.5 as functions of temperature, as determined from the NPD results for the ZrBe
2H
0.6, ZrBe
2H
1.2, and ZrBe
2H
1.5 samples listed in
Tables S3, S6 and S8. Data are plotted in a way that allows for a meaningful comparison of the changing dimensions of the structural hexagonal or quasi-hexagonal (in reference to the orthorhombic phases) ZrBe
2 template.
Figure 6a shows the temperature evolution of the distance
d between Zr planes for the three compounds. This is measured parallel to the crystallographic
c-direction for all phases except for orthorhombic ZrBe
2H, in which it is measured parallel to the crystallographic
b-direction (see
Figure 2).
Figure 6b shows the temperature evolution of the average Zr basal-plane area
A/ZrBe
2H
x formula unit (f.u.). This refers to the
ab basal plane for all phases except for orthorhombic ZrBe
2H, in which it refers to the
ac basal plane.
Figure 6c shows the temperature evolution of the average volume
V/ZrBe
2H
x f.u., where
V =
d ×
A. This is equivalent to the unit cell volume of the hexagonal
P6/
mmm phase and one-fourth and one-twelfth of the unit cell volumes of the respective orthorhombic
Imma and
Cmcm phases.
As alluded to in
Figure 5, The room-temperature lattice-parameter values for ZrBe
2, designated by the black diamonds in
Figure 6, are not much different than those for the ordered orthorhombic ZrBe
2H
0.67 phase, again indicating that the overall volume of the underlying ZrBe
2 template is largely able to accommodate the inclusion of this small amount of H without much volume expansion. In contrast, as the H content increases above
x = 0.67, the average volume/f.u. (seen in
Figure 5 and
Figure 6c) increases more significantly over the whole temperature range in a roughly linear fashion with
x, with
V expanding by around 0.8 (1) Å
3/H atom.
Figure 6a indicates that the distance
d between Zr planes noticeably expands with increasing
x above 0.67 to compensate for repulsive Be-H interactions, whereas the average basal-plane area
A (in
Figure 6b) noticeably contracts in response to attractive Zr-H interactions.
It is noteworthy that the orthorhombic-to-hexagonal phase change for ZrBe
2H near 460 K is marked by a step up in the
d spacing in
Figure 6a concomitant with a step down in the basal-plane area
A in
Figure 6b. No similarly obvious step behaviors are observed for ZrBe
2H
0.67 during its orthorhombic-to-hexagonal phase change near 490 K. Although the lack of clear step changes in the latter case may merely be an artifact of sparser data points, it may otherwise be an indicator of different H-site-spillover behaviors for the ordered orthorhombic ZrBe
2H and ZrBe
2H
0.67 phases as discussed earlier. In particular, the lack of H spillover into other neighboring empty crystallographic sites in orthorhombic ZrBe
2H up to the phase-transition temperature might well manifest itself by a relatively low
d spacing and a relatively high basal-plane area
A in this region due to the peculiar accommodating effects of the persistent double-chain H-atom ordering observed for this structure. As the transition occurs, the step change to a now random distribution of H atoms in the hexagonal structure could trigger clear step changes in lattice parameters. In contrast, for orthorhombic ZrBe
2H
0.67, a more gradual shift to H site disorder via site spillover with increasing temperature (as hinted by the NPD data) as the transition to complete disorder is approached may ultimately attenuate any transition-induced step-change effects in the lattice parameters.
As pointed out earlier for the ordered orthorhombic ZrBe
2H
0.67 and ZrBe
2H phases, H occupation of BeZr
3Be trigonal bipyramids result in planar contraction of the Zr
3 triads and expansion of the Be-Be separations. This is reflected structurally by
ab planar displacements of the associated Zr atoms and c-axis displacements of the associated Be atoms. These displacements should occur even in the hexagonal disordered ZrBe
2H
x structure, although in a disordered fashion, and this would be reflected by noticeable anisotropic behaviors in the associated Zr and Be displacement parameters. Indeed, the refined 15 K and 300 K anisotropic displacement parameters for ZrBe
2H
1.5 listed in
Table S2 indicate relatively large
ab basal-plane components compared to the c-axis component for Zr and a relatively a large c-axis component compared to the
ab basal-plane components for Be.
3.2. Neutron Vibrational Spectroscopy
Figure 7 shows the neutron vibrational spectra for the ZrBe
2D
1.5 and ZrBe
2H
x (
x = 0.6, 0.9, 1.2, 1.5) samples at both 10 K and room temperature (except for the ZrBe
2H
0.6 sample, where the gray spectrum was measured at 480 K). They were measured in neutron energy loss at superior resolution and intensity to the prior measurements reported in [
15]. The scattering features reported for ZrBe
2H
1.5 and ZrBe
2D
1.5 in [
15] are in line with the corresponding room-temperature spectra in
Figure 7, although there was no mention of observing the two highest-energy features clearly present in the current NV spectra.
The NV spectra reflect the H-weighted phonon densities of states (PDOSs) due to the overly dominant incoherent neutron scattering cross section for H compared to the other elements. Indeed, D, Be, and Zr all have similar scattering cross sections, but are all about an order of magnitude less than for H. Hence, the majority of scattering intensity in an NV spectrum reflects the various normal-mode vibrations involving H displacements. Nonetheless, there will be minor contributions from potential Be displacements due to its relatively low mass. Of course, in the non-H-containing ZrBe2D1.5 sample, the Be scattering contributions will be more noticeable in the spectrum with respect to those for D. In contrast, any contributions from Zr will be insignificant due to its relatively high mass.
Ideally at 0 K, all of the constituent atoms are in their vibrational ground states. At 10 K, this is still largely true, with only a very minor fraction of the atoms in their vibrational excited states. Thus, the 10 K spectra in
Figure 7 reflect normal-mode vibrational excitations predominantly from the ground states and contain both 1-phonon and 2-phonon (combination) modes. In contrast, the higher-temperature spectra reflect the normal-mode vibrational excitations from a mixed population of atoms, with one fraction in their ground states and another in their excited states. The spectra are thus more complicated due to a larger variety of resulting excitations and combination bands involving both up- and down-scattering as well as more severe attenuation by the Debye-Waller factor. Nonetheless, the higher-temperature spectra in
Figure 7 do reflect similar characteristics to the corresponding 10 K spectra, albeit more smeared in appearance.
The 10 K spectra are snapshots of all the low-temperature ordered structures present in the ZrBe
2H
x and ZrBe
2D
1.5 samples, and it is clear that the PDOSs for ZrBe
2H
x are markedly sensitive to the H content. A better understanding of the spectral features can be attained by first-principles phonon calculations. Because the low-temperature ordering in ZrBe
2H
1.5 and ZrBe
2D
1.5 is still unknown, no attempt was made to extract the DFT-simulated PDOS from possible ordered structural arrangements. Nonetheless, the simulated PDOSs for the two DFT-optimized, ordered orthorhombic ZrBe
2H and ZrBe
2H
0.67 structures, shown in
Figure 8 compared to their 10 K NV spectra, were found to greatly aid in the spectral assignments. The accompanying normal-mode animation files generated at the gamma point for the 0 K DFT-optimized structures are provided in the
Supplementary Materials. These ASCII files can be used with appropriate software [
36] to visualize the displacement amplitudes of the various unit-cell atoms for each normal-mode vibration. It should be noted, though, that the various gamma-point energies associated with particular vibrational modes may not exactly match the positions of the simulated peaks, since simulated spectra are averaged over the
Q-space accessed by the FANS instrument, not at the gamma point. Any dispersion of a mode in
Q-space may result in a shifting and complex broadening of the resulting spectral feature.
Based on the NPD results, the ZrBe
2H
0.9 sample is essentially a single-phase hydride with the orthorhombic ZrBe
2H structure. Although there is not perfect agreement with the DFT-simulated
Imma PDOS (which can be attributed to some minor lack of accuracy afforded by this level of DFT to describe the various interaction potentials involved), it is straightforward to assign the various NVS features to the vibrational features (designated by labelled arrows) as identified by DFT. Firstly, all the weak NVS features below ~70 meV are attributable to various Be-dominated normal-mode vibrations with usually little participation from H displacements. Slightly more intense features in this region, such as the small peak at ~69 meV associated with DFT feature (a) in
Figure 8, are due to Be-dominated modes with more significant H participation. For example, (a) in
Figure 8 represents Be displacements along the
c-direction (parallel to the Zr planes for
Imma symmetry) concomitant with small displacements of the H atoms along the
b-direction (perpendicular to the Zr planes). The strong NVS features above ~70 meV are associated with the six normal modes involving predominantly large-amplitude H displacements, with one in-phase and one out-of-phase normal mode (with respect to nearest-neighbor pairs of H atoms) along each of the three orthogonal directions. The 81.7 meV NVS feature associated with DFT feature (b) reflects the in-phase and out-of-phase modes involving H displacements along the
b-direction. The 114 meV NVS feature associated with DFT feature (c) reflects the two in-phase modes involving H displacements along the
a- and
c-directions (both parallel to the Zr planes). Finally, the highest NVS features between 130 meV and 150 meV associated with DFT feature (d) reflect the two out-of-phase modes involving H displacements along the
a- and
c-directions, with the mode along the
a-direction (along the zigzag H chain) associated with the higher-energy portion near 150 meV and that along the
c-direction (perpendicular to the zigzag H chain) associated with the lower-energy portion near 130 meV. Considerable normal-mode dispersion with
Q in this high-energy region is suggested by both the NVS and DFT spectra.
Because the NPD results indicated that the ZrBe
2H
0.6 sample was actually a mixed phase (i.e., orthorhombic ZrBe
2H
0.67 with a small fraction of orthorhombic ZrBe
2H at low temperature), the NV spectrum for orthorhombic ZrBe
2H
0.67 in
Figure 8 was isolated by subtracting an appropriately scaled ZrBe
2H
0.9 sample spectrum (representing orthorhombic ZrBe
2H) from the ZrBe
2H
0.6 sample spectrum. It should be noted that, even after the subtraction, a comparison of the extracted ZrBe
2H
0.67 spectrum in
Figure 8 with the parent ZrBe
2H
0.6 sample spectrum in
Figure 7 indicates very little qualitative difference.
Differences between the ZrBe
2H
0.67 spectrum and the DFT-simulated
Cmcm PDOS again reflect the limitations of this level of DFT to accurately describe the various interaction potentials involved. Moreover, the simulated PDOS is based on an optimized
Cmcm structure with full occupancies of the H1 and H2 sites. In reality, the actual refined structure indicated only a partial occupancy of the H2 site (~82% at 45 K). The fact that some of the H2 sites are randomly vacant likely results in the noticeably broadened scattering features compared to the sharp features of the simulated spectrum. Nonetheless, this does not prohibit using the simulated PDOS to identify the origin of the NV spectral features. As for ZrBe
2H, the NV scattering features below ~77 meV are attributable to Be-dominated normal modes as, e.g., evidenced by DFT feature (e) and lower-energy features in
Figure 8. There are twelve normal modes involving the large-amplitude H displacements of H1 and H2 atoms situated above 77 meV. This is twice as many as for ZrBe
2H, since there are now two different H sites to consider with their accompanying combinations of in-phase and out-of-phase motions instead of only one H site for ZrBe
2H. The 88 meV NVS shoulder associated with DFT feature (f) reflects two modes involving only H1 displacements along the
c-direction (perpendicular to the Zr planes for
Cmcm symmetry). The 106 meV NVS feature associated with DFT features (g) and (h) reflect the respective two in-phase and two out-of-phase modes involving H1 and H2 atoms along the
a-direction (parallel to the Zr planes and along the double-chain direction). The 118 meV NVS feature associated with DFT feature (i) reflect two modes involving only H2 displacements along the
c-direction. The 133 meV NVS feature associated with DFT feature (j) reflects the two in-phase modes involving H1 and H2 atoms along the
b-direction (parallel to the Zr planes and perpendicular to the double chain direction). Finally, the 150 meV NVS shoulder associated with DFT feature (k) reflects the two out-of-phase modes involving H1 and H2 atoms along the
b-direction.
The mode assignments for both ZrBe
2H and ZrBe
2H
0.67 make sense based on the structural details. The Be-dominated modes reside between ~45 meV to 75 meV, energies of this two-dimensional Be network consistent with those found in elemental Be [
37]. The H vibrational modes perpendicular to the Zr planes along the Be-H-Be axis of the H-occupied BeZr
3Be trigonal bipyramids tend to have lower energies. For ZrBe
2H
0.67, the H1- and H2-only modes (f) and (i) along the Be-H-Be axis indicate lower energies for the H1 atoms (with relatively larger Be-H bond distances) compared to the H2 atoms (with relatively smaller Be-H bond distances). The H vibrational modes within the Zr planes tend to have higher energies due to the relatively strong Zr-H bonding interactions, with the highest energies occurring for the out-of-phase vibrational modes, which are additionally affected by H-H coupling interactions during the opposing displacements of nearest-neighbor H atoms with close 2.1–2.2 Å separations in the structures. Interestingly, these high-energy scattering bands between ~125 meV and 165 meV associated with trigonally bonded H within the planar Zr
3 sites for the various ZrBe
2H
x phases match well with the broad range of vibrational energies for H in the Zr
4 tetrahedral sites of ZrH
x (
x = 0.54–2.0) [
38,
39] with comparable Zr-H bond distances (e.g., 2.08 Å for ZrH
2 at 297 K) [
40].
These observations allow us to assign the probable origins of the NV features for ZrBe
2H
1.5 in
Figure 7. In particular, the two higher-energy features near 123 meV and 147 meV are likely associated with the various respective in-phase and out-of-phase H vibrational modes parallel to the Zr planes, whereas the 90 meV shoulder and 107.5 meV feature are likely associated with various H vibrational modes perpendicular to the Zr planes. It should be noted that these assignments are different than postulated in the earlier NVS study [
15], since the higher-energy NV features in
Figure 7 were not detected in that study.
The ZrBe
2D
1.5 spectrum in
Figure 7 is seen to be in good agreement with that for ZrBe
2H
1.5. After rescaling the energy-loss values by a harmonic magnitude of
, the resulting red ZrBe
2D
1.5 spectrum in
Figure 7 closely mimics the ZrBe
2H
1.5 spectrum except for the low-energy feature near 68 meV, which is confirmation that the original 48 meV ZrBe
2D
1.5 feature is due to Be-dominated vibrational modes. Again, this feature is relatively enhanced compared to the D-related modes due to the similar neutron scattering cross sections for Be and D. This is analogous to the observed 52 meV ZrBe
2H
1.5 feature in
Figure 7, whose considerable intensity signals the likely involvement of some H displacements concomitant with the main Be displacements. (It should be noted that the weak ZrBe
2D
1.5 feature at 36 meV is an Al phonon artifact from the sample can and refrigerator cryoshields.)
It is interesting that the highest two harmonically rescaled D modes (originally located at ~89 meV and 105 meV) align very well with the ZrBe
2H
1.5 features at 123 meV and 147 meV, whereas the lower-energy shoulder and peak (originally located respectively at 69 meV and 79.5 meV) are about 8% and 5% higher after
rescaling than the corresponding ZrBe
2H
1.5 features at 90 meV and 107.5 meV. Although this may reflect some anharmonicity for the H potential well along the c-direction between Be atoms compared a more harmonic well along the
ab-plane within the Zr
3 trigonal sites, it may just be a consequence of some participation of the relatively low-mass Be atoms (m
Be = 9 g mol
−1) in the H optical vibrational modes along the
c-directed Be-H-Be axis. In such a case, the ratio of reduced masses for these modes in ZrBe
2D
1.5 and ZrBe
2H
1.5 would be smaller than the 2:1 ratio of masses for D and H alone and would indeed be manifested as a reduction in the otherwise harmonic
EH/
ED =
=
isotope shift. For example, the ratio of reduced masses between D and H modes, assuming the participation of one Be atom per D or H atom becomes 1.818. The expected harmonic energy-scaling factor for these particular
c-axis modes would now be
= 1.348, which is more in line with the spectral behavior in
Figure 7. Of course, this would not be as much of an issue for the higher-energy H vibrations within the Zr planes, since no Be participation would be expected in this orthogonal direction and the reduced-mass contributions from the much-higher-mass Zr atoms (
mZr = 91 g/mol
−1) would be much more insignificant, thus largely preserving the expected
harmonic rescaling factor for these modes, as observed.
Finally, we point out that the NV spectrum of the ZrBe
2H
1.2 sample in
Figure 7 is a combination of the spectra for the ZrBe
2H
1.5 and ZrBe
2H
0.9 samples, consistent with the two phases, ZrBe
2H
~1.3–1.4 and ZrBe
2H, observed by NPD. The lower-energy spectral contributions near 90 meV and 107.5 meV from the more minor ZrBe
2H
~1.3–1.4 component appear to be somewhat narrower than for ZrBe
2H
1.5, possibly because the relatively lower H content for ZrBe
2H
~1.3–1.4 results in mode-sharpening perturbations. The small differences in resolution between the two measured spectra cannot account for these changes in peak width.