Antiparasitic Activity of Oxindolimine–Metal Complexes against Chagas Disease
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of the Ligands
2.2. Syntheses of the Metal Complexes
2.3. Materials and Methods
2.4. Cells and Parasites
2.5. Viability Test in Mouse Peritoneal Macrophages (MTT Assay)
2.6. Effect of Compounds on the Trypomastigote Forms of T. cruzi
2.7. Evaluation of the Effect of Compounds on Amastigote Forms of T. cruzi
2.8. MTT Assay with Trypomastigote Parasites
3. Results and Discussion
3.1. Characterization and Stability of the Metal Complexes
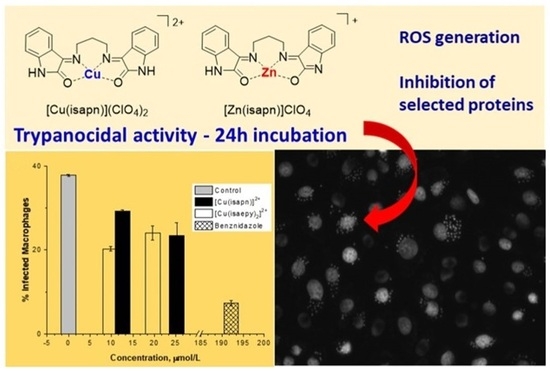
3.2. Evaluation of Trypanocidal Activity
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cruzain | a recombinant form of protein cruzipain, EC 3.4.22.51 |
| DPPH | α,α′-diphenyl-β-picrylhydrazyl |
| EPR | electron paramagnetic resonance |
| HGPRTs | hypoxanthine−guanine phosphoribosyl-transferases |
| isaepy | (E)-3-((2-(pyridin-2-yl)ethyl)imino)indolin-2-one; oxindolimine ligand obtained from isatin and 2-(2-aminoethyl)pyridine |
| isapn | (3E,3’E)-3,3’-(propane-1,3-diylbis(azaneylylidene)bis(indolin-2-one); oxindolimine ligand obtained from isatin and 1,3-diaminopropane |
| U2AF35 | host protein that binds to RNA at the polypyrimidine tract |
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 7 August 2023).
- Available online: https://dndi.org/diseases/chagas/ (accessed on 7 August 2023).
- Conners, E.E.; Vinetz, J.M.; Weeks, J.R.; Brouwer, K.C. A global systematic review of Chagas disease prevalence among migrants. Acta Tropica 2016, 156, 68–78. [Google Scholar] [CrossRef]
- Monge-Maillo, B.; Lopez-Velez, R. Challenges in the management of Chagas disease in Latin-American migrants in Europe. Clin. Microbiol. Infect. 2017, 23, 290–295. [Google Scholar] [CrossRef]
- Bern, C.; Messenger, L.A.; Whitman, J.D.; Maguire, J.H. Chagas disease in the United States: A public health approach. Clin. Microbiol. Rev. 2020, 33, e00023-19. [Google Scholar] [CrossRef]
- Trouiller, P.; Olliaro, P.; Torreele, E.; Orbinski, J.; Laing, R.; Ford, N. Drug development for neglected diseases: A deficient market and a public-health policy failure. Lancet 2002, 359, 2188–2194. [Google Scholar] [CrossRef]
- Horn, D. A profile of research on the parasitic trypanosomatids and the diseases they cause. PLoS Neg. Trop. Dis. 2022, 16, e0010040. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Kalesh, K. Defeating the trypanosomatid trio: Proteomics of the protozoan parasites causing neglected tropical diseases. RSC Med. Chem. 2020, 11, 625–645. [Google Scholar] [CrossRef]
- Barrett, M.P.; Burchmore, R.J.S.; Stich, A.; Lazzari, J.O.; Frasch, A.C.; Cazzulo, J.J.; Krishna, S. The trypanosomiases. Lancet 2003, 362, 1469–1480. [Google Scholar] [CrossRef]
- Martín-Escolano, J.; Marín, C.; Rosales, M.J.; Tsaousis, A.D.; Medina-Carmona, E.; Martín-Escolano, R. An Updated View of the Trypanosoma cruzi Life Cycle: Intervention Points for an Effective Treatment. ACS Infect. Dis. 2022, 8, 1107–1115. [Google Scholar] [CrossRef]
- Guedes, P.M.M.; Fietto, J.L.R.; Lana, M.; Bahia, M.T. Advances in Chagas Disease Chemotherapy. Anti.-Infect. Ag. Med. Chem. 2006, 5, 175–186. [Google Scholar] [CrossRef]
- Duschak, V.G.; Couto, A.S. An insight on targets and patented drugs for chemotherapy of Chagas disease. Recent Pat. Anti.-Infect. Drug Discov. 2007, 2, 19–51. [Google Scholar] [CrossRef]
- Njoroge, M.; Njuguna, N.M.; Mutai, P.; Ongarora, D.S.B.; Smith, P.W.; Chibale, K. Recent Approaches to Chemical Discovery and Development against Malaria and the Neglected Tropical Diseases Human African Trypanosomiasis and Schistosomiasis. Chem. Rev. 2014, 114, 11138–11163. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.O.; Faundez, M.; Morello, A.; Maya, J.D.; Tapia, R.A. Natural and Synthetic Naphthoquinones Active Against Trypanosoma Cruzi: An Initial Step Towards New Drugs for Chagas Disease. Curr. Med. Chem. 2011, 18, 144–161. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.V.; de Castro, S.L. The Trypanocidal Activity of Naphthoquinones: A Review. Molecules 2009, 14, 4570–4590. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.R.M.; Lima Leite, A.C.; Cardoso, M.V.O.; Srivastava, R.M.; Hernandes, M.Z.; Rabello, M.M.; da Cruz, L.F.; Ferreira, R.S.; de Simone, C.A.; Meira, C.S.; et al. Structural Design, Synthesis and Structure—Activity Relationships of Thiazolidinones with Enhanced Anti-Trypanosoma cruzi Activity. ChemMedChem 2014, 9, 177–188. [Google Scholar] [CrossRef]
- de Moraes Gomes, P.A.T.; de Oliveira Barbosa, M.; Santiago, E.F.; de Oliveira Cardoso, M.V.; Costa, N.T.C.; Hernandes, M.Z.; Rabello, M.M.; da Cruz, L.F.; Fer-reira, R.S.; de Simone, C.A.; et al. New 1,3-thiazole derivatives and their biological and ultrastructural effects on Trypanosoma cruzi. Eur. J. Med. Chem. 2016, 121, 387–398. [Google Scholar] [CrossRef]
- Bernatchez, J.A.; Kil, Y.-S.; da Silva, E.B.; Thomas, D.; McCall, L.I.; Wendt, K.L.; Souza, J.M.; Ackermann, J.; McKerrow, J.H.; Cichewicz, R.H.; et al. Identification of Leucinostatins from Ophiocordyceps sp. as Antiparasitic Agents against Trypanosoma cruzi. ACS Omega 2022, 7, 7675–7682. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Oswald, I.P.; Hieny, S.; James, S.L.; Sher, A. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, nitrogen oxide-mediated mechanism inhabitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 1992, 22, 2501–2506. [Google Scholar] [CrossRef]
- Alvarez, M.N.; Peluffo, G.; Piacenza, L.; Radi, R. Intraphagosomal Peroxynitrite as a Macrophage-derived Cytotoxin against Internalized Trypanosoma cruzi. J. Biol. Chem. 2011, 286, 6627–6640. [Google Scholar] [CrossRef]
- Maldonado, C.R.; Marín, C.; Olmo, F.; Huertas, O.; Quirós, M.; Sánchez-Moreno, M.; Rosales, M.J.; Salas, J.M. In Vitro and in Vivo Trypanocidal Evaluation of Nickel Complexes with an Azapurine Derivative against T. cruzi. J. Med. Chem. 2010, 53, 6964–6972. [Google Scholar] [CrossRef]
- Rettondin, A.R.; Carneiro, Z.A.; Gonçalves, A.C.R.; Ferreira, V.F.; Oliveira, C.G.; Lima, A.N.; Oliveira, R.J.; Albuquerque, S.; de Deflon, V.M.; Maia, P.I.; et al. Gold(III) complexes with ONS-Tridentate thiosemicarbazones: Toward selective trypanocidal drugs. Eur. J. Med. Chem. 2016, 120, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.; Salas, J.M.; Ana, B.; Caballero, A.B.; Esteban-Parra, G.M.; Méndez-Arriaga, J.M. Leishmanicidal and Trypanocidal Activity of Metal Complexes with 1,2,4-Triazolo[1,5-a]pyrimidines: Insights on their Therapeutic Potential against Leishmaniasis and Chagas Disease. Curr. Med. Chem. 2017, 24, 2796–2806. [Google Scholar]
- Santosa, D.; Parajón-Costa, B.; Rossi, M.; Caruso, F.; Benítez, D.; Varela, J.; Cerecetto, H.; González, M.; Gómez, N.; Caputto, M.E.; et al. Activity on Trypanosoma cruzi, erythrocytes lysis and biologically relevant physicochemical properties of Pd(II) and Pt(II) complexes of thiosemicarbazones derived from1-indanones. J. Inorg. Biochem. 2012, 117, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Gabbiani, C.; Messori, L.D.; Gambino, D. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives. Drug Discov. Today 2010, 15, 1070–1078. [Google Scholar] [CrossRef]
- Barbosa, M.I.F.; Corrêa, R.S.; de Oliveira, K.M.; Rodrigues, C.; Ellena, J.; Nascimento, O.R.; Rocha, V.P.C.; Nonato, F.R.; Macedo, T.S.; Barbosa-Filho, J.M.; et al. Antiparasitic activities of novel ruthenium/lapachol complexes. J. Inorg. Biochem. 2014, 136, 33–39. [Google Scholar] [CrossRef]
- Sarniguet, C.; Toloza, J.; Cipriani, M.; Lapier, M.; Vieites, M.; Toledano-Magaña, Y.; Otero, L. Water-Soluble Ruthenium Complexes Bearing Activity Against Protozoan Parasites. Biol. Trace Elem. Res. 2014, 159, 379–392. [Google Scholar] [CrossRef]
- Benítez, J.; Becco, L.; Correia, I.; Leal, S.M.; Guiset, H.; Costa Pessoa, J.; Lorenzo, J.; Tanco, S.; Escobar, P.; Moreno, V.; et al. Vanadium polypyridyl compounds as potential antiparasitic and antitumoral agents: New achievements. J. Inorg. Biochem. 2011, 105, 303–312. [Google Scholar] [CrossRef]
- Fricker, S.P.; Mosi, R.M.; Cameron, B.R.; Baird, I.; Zhu, Y.; Anastassov, V.; Cox, J.; Doyle, P.S.; Hansell, E.; Lau, G.; et al. Metal compounds for the treatment of parasitic diseases. J. Inorg. Biochem. 2008, 102, 1839–1845. [Google Scholar] [CrossRef]
- Glockzin, K.; Kostomiris, D.; Minnow, Y.V.T.; Suthagar, K.; Clinch, K.; Gai, S.; Buckler, J.N.; Schramm, V.L.; Tyler, P.C.; Meek, T.D. Kinetic Characterization, and Inhibition of Trypanosoma cruzi Hypoxanthine−Guanine Phosphoribosyltransferases. Biochemistry 2022, 61, 2088–2105. [Google Scholar] [CrossRef]
- Sánchez-Delgado, R.A.; Anzellotti, A. Metal Complexes as Chemotherapeutic Agents Against Tropical Diseases: Trypanosomiasis, Malaria and Leishmaniasis. Mini.-Rev. Med. Chem. 2004, 4, 23–30. [Google Scholar]
- Rajão, M.A.; Furtado, C.; Alves, C.L.; Passos-Silva, D.G.; de Moura, M.B.; Schamber-Reis, B.L.; Kunrath-Lima, M.; Zuma, A.A.; Vieira-da-Rocha, J.P.; Garcia, J.B.F.; et al. Unveiling Benznidazole’s mechanism of action through overexpression of DNA repair proteins in Trypanosoma cruzi. Environ. Mol. Mutagen. 2014, 55, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R. Sensitivity of parasites to free radical damage by antiparasitic drugs. Chem.-Biol. Interact. 1990, 73, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Goes, G.R.; Rocha, P.S.; Diniz, A.R.S.; Aguiar, P.H.N.; Machado, C.R.; Vieira, L.Q. Trypanosoma cruzi Needs a Signal Provided by Reactive Oxygen Species to Infect Macrophages. PLoS Neg. Trop. Dis. 2016, 10, e0004555. [Google Scholar] [CrossRef] [PubMed]
- Gachet-Castro, C.; Freitas-Castro, F.; Gonzáles-Córdova, R.A.; da Fonseca, C.I.K.; Gomes, M.D.; Ishikawa-Ankerhold, H.C.; Baqui, M.M.A. Modulation of the Host Nuclear Compartment by T. cruzi Uncovers Effects on Host Transcription and Splicing Machinery. Front. Cell. Infect. Microbiol. 2021, 11, 718028. [Google Scholar] [CrossRef]
- Tronchre, H.; Wang, J.; Fu, X.-D. A protein related to splicing factor U2AF35 that interacts with U2AF65 and SR proteins in splicing of pre-mRNA. Nature 1997, 388, 397–400. [Google Scholar] [CrossRef]
- de Paiva, R.E.F.; Vieira, E.G.; da Silva, D.R.; Wegermann, C.A.; da Costa Ferreira, A.M. Anticancer compounds based on isatin-derivatives: Strategies to ameliorate selectivity and efficiency. Front. Mol. Biosci. 2021, 7, 627272. [Google Scholar]
- de Moraes, J.; Dario, S.; Couto, R.A.A.; Pinto, P.L.S.; Da Costa Ferreira, A.M. Antischistosomal Activity of Oxindolimine-Metal Complexes. Antimicrob. Agents Chemother. 2015, 59, 6648–6652. [Google Scholar] [CrossRef]
- da Silveira, V.C.; Luz, J.S.; Oliveira, C.C.; Graziani, I.; Ciriolo, M.R.; Da Costa Ferreira, A.M. Double-strand DNA cleavage induced by oxindole-Schiff base copper(II) complexes with potential antitumor activity. J. Inorg. Biochem. 2008, 102, 1090–1103. [Google Scholar] [CrossRef]
- Katkar, P.; Coletta, A.; Castelli, S.; Sabino, G.L.; Couto, R.A.A.; Da Costa Ferreira, A.M.; Desideri, A. Effect of oxindolimine copper(II) and zinc(II) complexes on human topoisomerase I activity. Metallomics 2014, 6, 117–125. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Lima, L.; de Almeida, L.A.; Monteiro, J.; Moreno, C.J.G.; Nascimento, J.D.; de Araújo, R.F.; Mello, F.; Martins, L.P.A.; Graminha, M.A.S.; et al. Biological and Molecular Characterization of Trypanosoma cruzi Strains from Four States of Brazil. Am. J. Trop. Med. Hyg. 2018, 98, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Meth. Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Andrews, N.W.; Colli, W. Adhesion and interiorization of Trypanosoma cruzi in mammalian cells. J. Protozool. 1982, 29, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Miguel, R.B.; Petersen, P.A.D.; Gonzales-Zubiate, F.A.; Oliveira, C.C.; Kumar, N.; do Nascimento, R.R.; Petrilli, H.M.; Da Costa Ferreira, A.M. Inhibition of cyclin-dependent kinase CDK1 by oxindolimine ligands and corresponding copper and zinc complexes. J. Biol. Inorg. Chem. 2015, 20, 1205–1217. [Google Scholar] [CrossRef]
- Sakaguchi, U.; Addison, A.W. Spectroscopic and Redox Studies of Some Copper(II) Complexes with Biomimetic Donor Atoms: Implications for Protein Copper Centres. J. Chem. Soc. Dalton Trans. 1979, 1979, 600–608. [Google Scholar] [CrossRef]
- Da Costa Ferreira, A.M.; Petersen, P.A.D.; Petrilli, H.M.; Ciriolo, M.R. Molecular basis for anticancer and antiparasite activities of copper-based drugs. In Redox-Active Therapeutics; Batinic-Haberle, I., Reboucas, J.S., Spasojevic, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; Chapter. 12; pp. 287–309. [Google Scholar]
- Rozga, M.; Sokołowska, M.; Protas, A.M.; Bal, W. Human serum albumin coordinates Cu(II) at its N-terminal binding site with 1 pM affinity. J. Biol. Inorg. Chem. 2007, 12, 913–918. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Harvey, I.; Bunyan, K.E.; Stewart, A.J.; Sleep, D.; Harrison, D.J.; Berezenko, S.; Sadler, P.J. Structure, properties, and engineering of the major zinc binding site on human albumin. J. Biol. Chem. 2009, 284, 23116–23124. [Google Scholar] [CrossRef]
- Ciccarelli, A.B.; Frank, F.M.; Puente, V.; Malchiodi, E.L.; Batle, A.; Lombardo, M.E. Antiparasitic effect of vitamin B12 on T. cruzi. Antimicrob. Ag. Chemother. 2012, 56, 5315–5320. [Google Scholar] [CrossRef]
- Caballero, A.B.; Rodriguez-Diéguez, A.; Quirós, M.; Salas, J.M.; Huertas, O.; Ramírez-Macías, I.; Olmo, F.; Marín, C.; Chaves-Lemaur, G.; Gutierrez-Sánchez, R.; et al. Triazolopyrimidine compounds containing first-row transition metals and their activity against the neglected infectious Chagas disease and Leishmaniasis. Eur. J. Med. Chem. 2014, 85, 526–534. [Google Scholar] [CrossRef]
- Paixão, D.A.; Lopes, C.D.; Carneiro, Z.A.; Sousa, L.M.; de Oliveira, L.P.; Lopes, N.P.; Pivatto, M.; Chaves, J.D.S.; de Almeida, M.V.; Ellena, J.; et al. In vitro anti-Trypanosoma cruzi activity of ternary copper(II) complexes and in vivo evaluation of the most promising complex. Biomed. Pharmacother. 2019, 109, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.S.; da Silva, M.M.; Graminha, A.E.; Meira, C.S.; dos Santos, J.A.F.; Moreira, D.R.M.; Soares, M.B.P.; von Poelhsitz, G.; Castellano, E.E.; Bloch, C., Jr.; et al. Ruthenium(II) complexes of 1,3-thiazolidine-2-thione: Cytotoxicity against tumor cells and anti-Trypanosoma cruzi activity enhanced upon combination with benznidazole. J. Inorg. Biochem. 2016, 156, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Maia, P.I.D.S.; Carneiro, Z.A.; Lopes, C.D.; Oliveira, C.G.; Silva, J.S.; de Albuquerque, S.; Hagenbach, A.; Gust, R.; Deflon, V.M.; Abram, U. Organometallic gold(III) complexes with hybrid SNS-donating thiosemicarbazone ligands: Cytotoxicity and anti-Trypanosoma cruzi activity. Dalton Trans. 2017, 46, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Muelas-Serrano, S.; Nogal-Ruiz, J.J.; Gómez-Barrio, A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitol. Res. 2000, 86, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Merritt, C.; Silva, L.E.; Tanner, A.L.; Stuart, K.; Pollastri, M.P. Kinases as Druggable Targets in Trypanosomatid Protozoan Parasites. Chem. Rev. 2014, 114, 11280–11304. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.B.; Dall, E.; Briza, P.; Brandstetter, H.; Ferreira, R.S. Cruzain structures: Apocruzain and cruzain bound to S-ethylthiomethanesulfonate and implications for drug design. Acta Cryst. 2019, F75, 419–427. [Google Scholar]









| Complexes at Different pHs | g⊥ | g// | A//, G | A//,* 10−4cm−1 | g///A// cm |
|---|---|---|---|---|---|
| [Cu(isaepy)H2O] pH = 3, keto-form | 2.086 | 112 | 127 | 191 | |
| pH = 7 | 2.058 | 2.246 | 173 | 181 | 124 |
| pH = 10, enol-form | 2.059 | 2.252 | 177 | 186 | 121 |
| [Cu(isapn)] pH = 4, keto-keto form | 2.101 | 2.445 | 115 | 131 | 187 |
| pH = 7, keto-enol form | 2.112 | 2.256 | 186 | 196 | 115 |
| pH = 10, enol-enol form | 2.092 | 2.262 | 184 | 194 | 116 |
| IC50 µmol/L | [Cu(isapn)] (ClO4)2 | [Cu(isaepy)2] (ClO4)2 | [Zn(isapn)] ClO4 | [Zn(isaepy)Cl2] |
|---|---|---|---|---|
| 24 h | 15.5 ± 5.5 | 10.7 ± 3.8 | 32.9 ± 14.1 | 80.2 ± 52.6 |
| 48 h | 2.7 ± 1.0 | 3.0 ± 1.0 | 11.3 ± 3.6 | 56.2 ± 23.0 |
| LC50 µmol/L | [Cu(isapn)](ClO4)2 | [Cu(isaepy)2](ClO4)2 | [Zn(isapn)]ClO4 | [Zn(isaepy)Cl2] |
|---|---|---|---|---|
| 24 h | 73.3 ± 10.4 | 39.1 ± 3.5 | 183.8 ± 39.9 | 162.8 ± 18.8 |
| 48 h | 31.3 ± 14.0 | 16.2 ± 5.2 | 138.9 ± 23.8 | 177.8 ± 25.0 |
| IC50 (μM) after 24 h Incubation | IC50 (μM) after 48 h Incubation | |||||
|---|---|---|---|---|---|---|
| Complex | Macrophages | Trypomastigotes | S.I. | Macrophages | Trypomastigotes | S.I. |
| [Cu(isapn)] (ClO4)2 1 | 73.3 ± 10.4 | 15.5 ± 5.5 | 4.8 | 31.3 ± 14.0 | 2.7 ± 1.0 | 11.6 |
| [Zn(isapn)] ClO4 2 | 183.8 ± 39.9 | 32.9 ± 14.1 | 5.6 | 138.9 ± 23.8 | 11.3 ± 3.6 | 12.4 |
| [Cu(isaepy)2] (ClO4)2 5 | 39.1 ± 3.5 | 10.7 ± 3.8 | 3.7 | 16.2 ± 5.2 | 3.0 ± 1.0 | 5.4 |
| [Zn(isaepy)Cl2] 4 | 162.8 ± 18,8 | 80.2 ± 52.6 | 2.0 | 177.8 ± 25 | 56.2 ± 23 | 3.2 |
| Benznidazole # | 30.3 ± 2.83 | 2.7 | ||||
| Complexes | IC50 (μM) Trypomastigotes | Selective Index (S.I.) | Incubation Time |
|---|---|---|---|
| [Cu(dmtp)4(H2O)2] (ClO4)2 dmtp = 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine | 25.4 ± 2.3 | 16.5 | 72 h a |
| [Zn(dmtp)2(H2O)4] (ClO4)2 | 19.2 ± 1.1 | 3.8 | 72 h a |
| [Cu(4-MH)(dmb)(ClO4)2]∙2H2O 4-MH = 4-methoxybenzhydrazide; dmb = 4-4’-dimethoxy-2-2’-bipyridine | 14.0 | 12.9 | 72 h b |
| trans-[Ru(tzdt)(PPh3)2(bipy)]PF6 tzdtH = 1,3-thiazolidine-2-thione | 0.01 | 34 | 24 h c |
| [AuIII(Hdamp)(L1)]NO3 (4-NO3) Hdamp = dimethylaminomethylphenyl | 16.9 | 5.1 | 48 h d |
| [Pt(HL1)(L1)]Cl # L1 = thiosemicarbazone derivative of 1-indanone | (8.7) | (8.8) | 120 h e (Epimastigote form) |
| [Pd(HL2)(L2)]Cl # L2 = thiosemicarbazone derivative of 1-indanone | (2.3) | (9.5) | 120 h e (Epimastigote form) |
| Complexes | Trypomastigotes (Neubauer Chamber) | Trypomastigotes (MTT) | Correlation (Neubauer Chamber/MTT) |
|---|---|---|---|
| [Cu(isapn)](ClO4)2 | 15.5 ± 5.5 µM | 6.11 ± 0.44 µM | 2.54 |
| [Cu(isaepy)2](ClO4)2 | 10.7 ± 3.8 µM | ||
| [Cu(isaepy)H2O]ClO4 | 1.37 ± 0.12 µM | 7.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portes, M.C.; Ribeiro, G.A.; Sabino, G.L.; De Couto, R.A.A.; Vieira, L.Q.; Alves, M.J.M.; Da Costa Ferreira, A.M. Antiparasitic Activity of Oxindolimine–Metal Complexes against Chagas Disease. Inorganics 2023, 11, 420. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110420
Portes MC, Ribeiro GA, Sabino GL, De Couto RAA, Vieira LQ, Alves MJM, Da Costa Ferreira AM. Antiparasitic Activity of Oxindolimine–Metal Complexes against Chagas Disease. Inorganics. 2023; 11(11):420. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110420
Chicago/Turabian StylePortes, Marcelo Cecconi, Grazielle Alves Ribeiro, Gustavo Levendoski Sabino, Ricardo Alexandre Alves De Couto, Leda Quércia Vieira, Maria Júlia Manso Alves, and Ana Maria Da Costa Ferreira. 2023. "Antiparasitic Activity of Oxindolimine–Metal Complexes against Chagas Disease" Inorganics 11, no. 11: 420. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110420