Effect of Metal Environment and Immobilization on the Catalytic Activity of a Cu Superoxide Dismutase Mimic
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Complex
2.2. Synthesis and Characterization of Modified Mesoporous Silicas
2.2.1. Synthesis of Cu-pypntriazole@SBA-15 and Cu-pypntriazole@OP-MS
2.2.2. Textural Properties and Morphology of the Mesoporous Materials
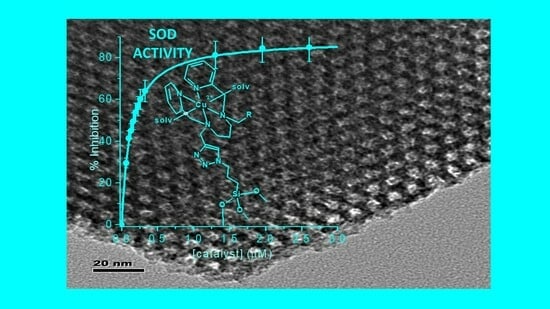
2.3. SOD Activity Studies
3. Materials and Methods
3.1. Synthesis of Ligands, Complexes, and Hybrid Materials
3.1.1. Synthesis of [Cu(pypapn)(ClO4)2]
3.1.2. Synthesis of Azidopropyl Functionalized Silicas N3pn@SBA-15 and N3pn@OP-MS
3.1.3. Synthesis of Cu-Pypntriazole@SBA-15 and Cu-Pypntriazole@OP-MS
3.1.4. Synthesis of Encapsulated Catalyst Cu-py2pn@SBA-15
3.2. Analytical and Physical Measurements
3.3. Crystal Data Collection and Refinement
3.4. Indirect SOD Assay
3.5. Preparation of Potassium Superoxide Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.A.; Cabelli, D.E. Superoxide dismutases—A review of the metal-associated mechanistic variations. Biochim. Biophys. Acta 2010, 1804, 263–274. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Reboucas, J.S.; Spasojević, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox. Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef]
- Riley, D.P. Functional Mimics of Superoxide Dismutase Enzymes as Therapeutic Agents. Chem. Rev. 1999, 99, 2573–2587. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.-F.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef]
- Tainer, J.A.; Getzoff, E.D.; Beem, K.M.; Richardson, J.S.; Richardson, D.C. Determination and analysis of the 2 Å structure of copper, zinc superoxide dismutase. J. Mol. Biol. 1982, 160, 181–217. [Google Scholar] [CrossRef]
- Richezzi, M.; Ferreyra, J.; Puzzolo, J.; Milesi, L.; Palopoli, C.M.; Moreno, D.M.; Hureau, C.; Signorella, S.R. Versatile Activity of a Copper(II) Complex Bearing a N4-Tetradentate Schiff Base Ligand with Reduced Oxygen Species. Eur. J. Inorg. Chem. 2022, 2022, e202101042. [Google Scholar] [CrossRef]
- Lange, J.; Elias, H.; Paulus, H.; Müller, J.; Weser, U. Copper(II) and Copper(I) complexes with an open-chain N4 Schiff base ligand modeling CuZn superoxide dismutase: Structural and spectroscopic characterization and kinetics of electron transfer. Inorg. Chem. 2000, 39, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Felix, K.; Maichle, C.; Lengfelder, E.; Strähle, J.; Weser, U. Phenyl-Substituted Copper Di-Schiff Base, a Potent CuZn Superoxide Dismutase Mimic Surviving Competitive Biochelation. Inorg. Chim. Acta 1995, 233, 11–19. [Google Scholar] [CrossRef]
- Pap, J.S.; Kripli, B.; Bors, I.; Bogáth, D.; Giorgi, M.; Kaizer, J.; Speier, G. Transition Metal Complexes Bearing Flexible N3 or N3O Donor Ligands: Reactivity toward Superoxide Radical Anion and Hydrogen Peroxide. J. Inorg. Biochem. 2012, 117, 60–70. [Google Scholar] [CrossRef]
- Patriarca, M.; Daier, V.; Camí, G.; Pellegri, N.; Rivière, E.; Hureau, C.; Signorella, S. Biomimetic Cu, Zn and Cu2 Complexes Inserted in Mesoporous Silica as Catalysts for Superoxide Dismutation. Microporous Mesoporous Mater. 2019, 279, 133–141. [Google Scholar] [CrossRef]
- Müller, J.; Schübl, D.; Maichle-Mössmer, C.; Strähle, J.; Weser, U. Structure—Function Correlation of Cu (II)-and Cu (I)-Di-Schiff-Base Complexes during the Catalysis of Superoxide Dismutation. J. Inorg. Biochem. 1999, 75, 63–69. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Fernandes, C.; Melo, K.V.; Ferreira, S.S.; Lessa, J.A.; Franco, R.W.A.; Schenk, G.; Pereira, M.D.; Horn, A., Jr. Iron, Copper, and Manganese Complexes with in Vitro Superoxide Dismutase and/or Catalase Activities That Keep Saccharomyces Cerevisiae Cells Alive under Severe Oxidative Stress. Free Radic. Biol. Med. 2015, 80, 67–76. [Google Scholar] [CrossRef]
- Mekhail, M.A.; Smith, K.J.; Freire, D.M.; Pota, K.; Nguyen, N.; Burnett, M.E.; Green, K.N. Increased Efficiency of a Functional SOD Mimic Achieved with Pyridine Modification on a Pyclen-Based Copper(II) Complex. Inorg. Chem. 2023, 62, 5415–5425. [Google Scholar] [CrossRef]
- Green, K.N.; Pota, K.; Tircso, G.; Gogolak, R.A.; Kinsinger, O.; Davda, C.; Blain, K.; Brewer, S.M.; Gonzalez, P.; Johnston, H.M.; et al. Dialing in on pharmacological features for a therapeutic antioxidant small molecule. Dalton Trans. 2019, 48, 12430–12439. [Google Scholar] [CrossRef]
- Policar, C.; Bouvet, J.; Bertrand, H.C.; Delsuc, N. SOD mimics: From the tool box of the chemists to cellular studies. Curr. Opin. Chem. Biol. 2022, 67, 102109. [Google Scholar] [CrossRef]
- Martinez-Camarena, Á.; Sanchez-Murcia, P.A.; Blasco, S.; Gonzalez, L.; Garcia-España, E. Unveiling the reaction mechanism of novel copper N-alkylated tetra-azacyclophanes with outstanding superoxide dismutase activity. Chem. Commun. 2020, 56, 7511–7514. [Google Scholar] [CrossRef]
- Vaughn, B.A.; Brown, A.M.; Ahn, S.H.; Robinson, J.R.; Borosm, E. Is Less More? Influence of the Coordination Geometry of Copper(II)Picolinate Chelate Complexes on Metabolic Stability. Inorg. Chem. 2020, 59, 16095–16108. [Google Scholar] [CrossRef]
- Smits, N.W.G.; van Dijk, B.; de Bruin, I.; Groeneveld, S.L.T.; Siegler, M.A.; Hetterscheid, D.G.H. Influence of Ligand Denticity and Flexibility on the Molecular Copper Mediated Oxygen Reduction Reaction. Inorg. Chem. 2020, 59, 16398–16409. [Google Scholar] [CrossRef]
- Stanek, J.; Hoffmann, A.; Herres-Pawlis, S. Renaissance of the entatic state principle. Coord. Chem. Rev. 2018, 365, 103–121. [Google Scholar] [CrossRef]
- Falcone, E.; Hureau, C. Redox processes in Cu-binding proteins: The “in-between” states in intrinsically disordered peptides. Chem. Soc. Rev. 2023, 52, 6595–6600. [Google Scholar] [CrossRef]
- Uzal-Varela, R.; Patinec, V.; Tripier, R.; Valencia, L.; Maneiro, M.; Canle, M.; Platas-Iglesias, C.; Esteban-Gómez, D.; Iglesias, E. On the dissociation pathways of copper complexes relevant as PET imaging agents. J. Inorg. Biochem. 2022, 236, 111951. [Google Scholar] [CrossRef]
- Mohammadnezhad, G.; Amirian, A.M.; Plass, H.G.W.; Sandleben, A.; Schäfer, S.; Klein, A. Redox Instability of Copper(II) Complexes of a Triazine-Based PNP Pincer. Eur. J. Inorg. Chem. 2021, 2021, 1140–1151. [Google Scholar] [CrossRef]
- Mureseanu, M.; Filip, M.; Bleotu, I.; Spinu, C.I.; Marin, A.H.; Matei, I.; Parvulescu, V. Cu(II) and Mn(II) Anchored on Functionalized Mesoporous Silica with Schiff Bases: Effects of Supports and Metal–Ligand Interactions on Catalytic Activity. Nanomaterials 2023, 13, 1884. [Google Scholar] [CrossRef]
- Isa, E.D.M.; Ahmad, H.; Rahman, M.B.A.; Gill, M.R. Progress in Mesoporous Silica Nanoparticles as Drug Delivery Agents for Cancer Treatment. Pharmaceutics 2021, 13, 152. [Google Scholar]
- Cadavid-Vargas, J.F.; Arnal, P.M.; Sepúlveda, R.D.M.; Rizzo, A.; Soria, D.B.; Di Virgilio, A.L. Copper complex with sulfamethazine and 2,2′-bipyridine supported on mesoporous silica microspheres improves its antitumor action toward human osteosarcoma cells: Cyto- and genotoxic effects. Biometals 2019, 32, 21–32. [Google Scholar] [CrossRef]
- Donato, L.; Atoini, Y.; Prasetyanto, E.A.; Chen, P.; Rosticher, C.; Bizarri, C.; Rissansen, K.; De Cola, L. Selective encapsulation and enhancement of the emission properties of a luminescent Cu(I) complex in mesoporous silica. Helvetica Chim. Acta 2018, 101, e1700273. [Google Scholar] [CrossRef]
- Richezzi, M.; Palopoli, C.; Pellegri, N.; Hureau, C.; Signorella, S.R. Synthesis, characterization and superoxide dismutase activity of a biomimetic Mn(III) complex covalently anchored to mesoporous silica. J. Inorg. Biochem. 2022, 237, 112026. [Google Scholar] [CrossRef] [PubMed]
- Pajchel, L.; Kolodziejski, W. Synthesis and characterization of MCM-48/hydroxyapatite composites for drug delivery: Ibuprofen incorporation, location and release studies. Mater. Sci. Eng. C 2018, 91, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Qu, L.; Kan, G.; Zhang, T.; Shen, J.; Li, Y.; Yang, J.; Niu, Y.; Xiao, Z.; et al. Reactive mesoporous silica nanoparticles loaded with limonene for improving physical and mental health of mice at simulated microgravity condition. Bioact. Mater. 2020, 5, 1127–1137. [Google Scholar] [CrossRef]
- Wang, L.S.; Wu, L.C.; Lu, S.Y.; Chang, L.L.; Teng, I.T.; Yang, C.M.; Ho, J.A. Biofunctionalized Phospholipid-Capped Mesoporous Silica Nanoshuttles for Targeted Drug Delivery: Improved Water Suspensibility and Decreased Nonspecific Protein Binding. ACS Nano 2010, 4, 4371–4379. [Google Scholar] [CrossRef]
- Martinez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef]
- Ha, S.W.; Viggeswarapu, M.; Habib, M.M.; Beck, G.R., Jr. Bioactive effects of silica nanoparticles on bone cells are size, surface, and composition dependent. Acta Biomater. 2018, 82, 184–196. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.J. Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications. Chem. Rev. 2019, 119, 1666–1762. [Google Scholar] [CrossRef]
- Freire, C.; Pereira, C.; Rebelo, S. Green oxidation catalysis with metal complexes: From bulk to nano recyclable hybrid catalysts. Catalysis 2012, 24, 116–203. [Google Scholar]
- Rana, B.S.; Jain, S.L.; Singh, B.; Bhaumik, A.; Sain, B.; Sinha, A.K. Click on silica: Systematic immobilization of Co(II)Schiff bases to the mesoporous silicavia click reaction and their catalytic activity for aerobic oxidation of alcohols. Dalton Trans. 2010, 39, 7760–7767. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Hosseini, M.; Mortazavi-Manesh, A. Manganese(III) porphyrin anchored onto magnetic nanoparticles via “Click” reaction: An efficient and reusable catalyst for the heterogeneous oxidation of alkenes and sulfides. Inorg. Chem. Commun. 2019, 107, 107495. [Google Scholar] [CrossRef]
- Richezzi, M.; Signorella, S.; Palopoli, C.; Pellegri, N.; Hureau, C.; Signorella, S.R. The Critical Role of Ligand Flexibility on the Activity of Free and Immobilized Mn Superoxide Dismutase Mimics. Inorganics 2023, 11, 359. [Google Scholar] [CrossRef]
- Pat McCurdie, M.; Belfiore, L.A. Spectroscopic analysis of transition-metal coordination complexes based on poly(4-vinylpyridine) and dichlorotricarbonylruthenium(II). Polymer 1999, 40, 2889–2902. [Google Scholar] [CrossRef]
- Subramanian, P.S.; Suresh, E.; Dastidar, P.; Waghmode, S.; Srinivas, D. Conformational Isomerism and Weak Molecular and Magnetic Interactions in Ternary Copper(II) Complexes of [Cu(AA)L’]ClO4·nH2O, Where AA = L-Phenylalanine and L-Histidine, L’ = 1,10-Phenanthroline and 2,2-Bipyridine, and n = 1 or 1.5: Synthesis, Single-Crystal X-ray Structures, and Magnetic Resonance Investigations. Inorg. Chem. 2001, 40, 4291–4301. [Google Scholar]
- Muthuramalingam, S.; Anandababu, K.; Velusamy, M.; Mayilmurugan, R. Benzene Hydroxylation by Bioinspired Copper(II) Complexes: Coordination Geometry versus Reactivity. Inorg. Chem. 2020, 59, 5918–5928. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Malvi, B.; Sarkar, B.R.; Pati, D.; Mathew, R.; Ajithkumarb, T.G.; Sen Gupta, S. “Clickable” SBA-15 mesoporous materials: Synthesis, characterization and their reaction with alkynes. J. Mater. Chem. 2009, 19, 1409–1416. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.J. Direct Synthesis of Hybrid Organic-Inorganic Nanoporous Silica by a Neutral Amine Assembly Route: Structure-Function Control by Stoichiometric Incorporation of Organosiloxane Molecules. Chem. Mater. 2000, 12, 188–196. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Frederichson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Taghavimoghaddam, J.; Knowles, G.P.; Chaffee, A.L. SBA-15 supported cobalt oxide species: Synthesis, morphology and catalytic oxidation of cyclohexanol using TBHP. J. Mol. Catal. A Chem. 2013, 379, 277–286. [Google Scholar] [CrossRef]
- Beauchamps, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Liao, Z.-R.; Zheng, X.-F.; Luo, B.-S.; Shen, L.-R.; Li, D.-F.; Liu, H.-L.; Zhao, W. SOD-like activities of manganese-containing complexes with N,N,N,N-tetrakis(2-benzimidazolyl methyl)-1,2-ethanediamine (EDTB). Polyhedron 2001, 20, 2813–2821. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Shang, D.-J.; Wang, X.-L.; You, Z.-L.; Li, H.-B. Antihyperglycemic and neuroprotective effects of one novel Cu–Zn SOD mimetic. Bioorg. Med. Chem. Lett. 2011, 21, 4320–4324. [Google Scholar] [CrossRef]
- You, Z.-L.; Ni, L.-L.; Hou, P.; Zhang, J.-C.; Wang, C. Synthesis, Crystal Structures, and Superoxide Dismutase Activity of Two Isostructural Copper(II)—Zinc(II) Complexes Derived from N,N′-Bis (4-Methoxysalicylidene) Cyclohexane-1, 2-Diamine. J. Coord. Chem. 2010, 63, 515–523. [Google Scholar] [CrossRef]
- Ivanović-Burmazović, I.; Filipović, M.R. Chapter 3—Reactivity of manganese superoxide dismutase mimics toward superoxide and nitric oxide: Selectivity versus cross-reactivity. Adv. Inorg. Chem. 2012, 64, 53–95. [Google Scholar]
- Diószegi, R.; Bonczidai-Kelemen, D.; Bényei, A.C.; May, N.V.; Fábián, I.; Lihi, N. Copper(II) Complexes of Pyridine-2,6-dicarboxamide Ligands with High SOD Activity. Inorg. Chem. 2022, 61, 2319–2332. [Google Scholar] [CrossRef]
- Bagchi, R.N.; Bond, A.M.; Scholz, F.; Stösser, R. Characterization of the ESR spectrum of the superoxide anion in the liquid phase. J. Am. Chem. Soc. 1989, 111, 8270–8271. [Google Scholar] [CrossRef]
- Ebralidze, I.I.; Leitus, G.; Shimon, L.J.W.; Wang, Y.; Shaik, S.; Neumann, R. Structural variability in manganese(II) complexes of N,N’-bis(2-pyridinylmethylene) ethane (and propane) diamine ligands. Inorg. Chim. Acta 2009, 362, 4713–4720. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Villarroel Rocha, J.; Barrera, D.; Sapag, K. Improvement in the Pore Size Distribution for Ordered Mesoporous Materials with Cylindrical and Spherical Pores Using the Kelvin Equation. Top. Catal. 2011, 54, 121–134. [Google Scholar] [CrossRef]
- Bruker, APEX4 v2022.10-1; Bruker AXS Inc.: Madison, WI, USA, 2022.
- Bruker, SAINT V8.40B; Bruker AXS Inc.: Madison, WI, USA, 2019.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. ORTEP3 for Windows. J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Hyland, K.; Auclair, C. The formation of superoxide radical anions by a reaction between O2, OH− and dimethyl sulfoxide. Biochem. Biophys. Res. Commun. 1981, 102, 531–537. [Google Scholar] [CrossRef]










| SBET (m2 g−1) | VμP (cm3 g−1) | VMP (cm3 g−1) | VTP (cm3 g−1) | wP (nm) | mmol Complex/100 g Material | |
|---|---|---|---|---|---|---|
| SBA-15 | 641 | 0.03 | 0.64 | 0.79 | 4.9 | - |
| Cu-pypntriazole@SBA-15 | 310 | 0.00 | 0.34 | 0.41 | 3.7 | 24 |
| Cu-py2pn@SBA-15 | 501 | 0.00 | 0.57 | 0.71 | 4.4 | 9.8 |
| N3pn@OP-MS | 362 | 0.00 | 0.41 | 0.47 | 4.8 | - |
| Cu-pypntriazole@OP-MS | 433 | 0.00 | 0.40 | 0.46 | 4.0 | 18 |
| Entry | Catalyst | Ligand Donor Sites | kMcF (106 M−1 s−1) | E1/2 (V vs. SCE) | Ref. |
|---|---|---|---|---|---|
| 1 | [Cu(PuPy)]2+ | N4 | 23.6 | - | [9] |
| 2 | [Cu(MPBMPA)Cl2] | N3 | 21.2 | −0.471 | [10] |
| 3 | [Cu(pypapn)]2+ | N4 | 12.6 | −0.22 (Epc) | This work |
| 4 | [Cu(PBMPA)Cl] | N3O | 12.5 | 0.213 | [10] |
| 5 | [CuZn(dien)2(μ-Im)(ClO4)2]+ | N3NIm | 6.46 | −0.89 (Epc) | [11] |
| 6 | [Cu(Pu-6-MePy)(H2O)]2+ | N4 | 6.3 | - | [12] |
| 7 | [Cu(py2pn)]2+ | N4 | 4.05 | −0.044 | [7] |
| 8 | [Cu(PClNOL)Cl]+ | N3O | 3.3 | −0.416 | [13] |
| 9 | [Cu(5-EtO-salpn)ZnCl2] | N2O2 | 2.1 | - | [49] |
| 10 | [Cu(4-OMe-salchda)ZnCl2] | N2O2 | 0.87 | - | [50] |
| 11 | [salpnCuZnCl2] | N2O2 | 0.85 | −0.689 | [11] |
| 12 | CuZnSOD | N4 | 2000 | 0.156 | [2] |
| Immobilized Catalyst | kMcF (106 M−1 s−1) | ||||
| 13 | Cu-pypntriazole@SBA-15 | 14.2 | |||
| 14 | Cu-pypntriazole@OP-MS | 13.3 | |||
| 15 | Cu-py2pn@SBA-15 | 6.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richezzi, M.; Ferreyra, J.; Signorella, S.; Palopoli, C.; Terrestre, G.; Pellegri, N.; Hureau, C.; Signorella, S.R. Effect of Metal Environment and Immobilization on the Catalytic Activity of a Cu Superoxide Dismutase Mimic. Inorganics 2023, 11, 425. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110425
Richezzi M, Ferreyra J, Signorella S, Palopoli C, Terrestre G, Pellegri N, Hureau C, Signorella SR. Effect of Metal Environment and Immobilization on the Catalytic Activity of a Cu Superoxide Dismutase Mimic. Inorganics. 2023; 11(11):425. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110425
Chicago/Turabian StyleRichezzi, Micaela, Joaquín Ferreyra, Sharon Signorella, Claudia Palopoli, Gustavo Terrestre, Nora Pellegri, Christelle Hureau, and Sandra R. Signorella. 2023. "Effect of Metal Environment and Immobilization on the Catalytic Activity of a Cu Superoxide Dismutase Mimic" Inorganics 11, no. 11: 425. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics11110425