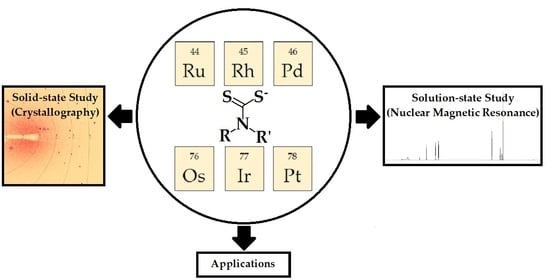
Dithiocarbamate Complexes of Platinum Group Metals: Structural Aspects and Applications
Abstract
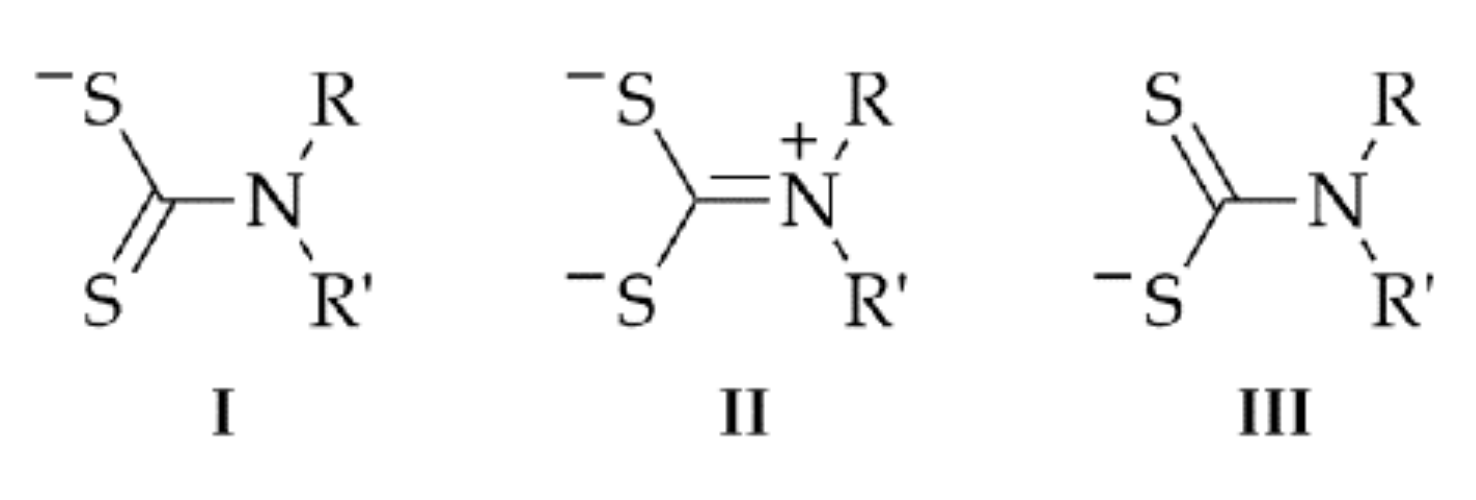
:1. Introduction
2. Methods
3. Results
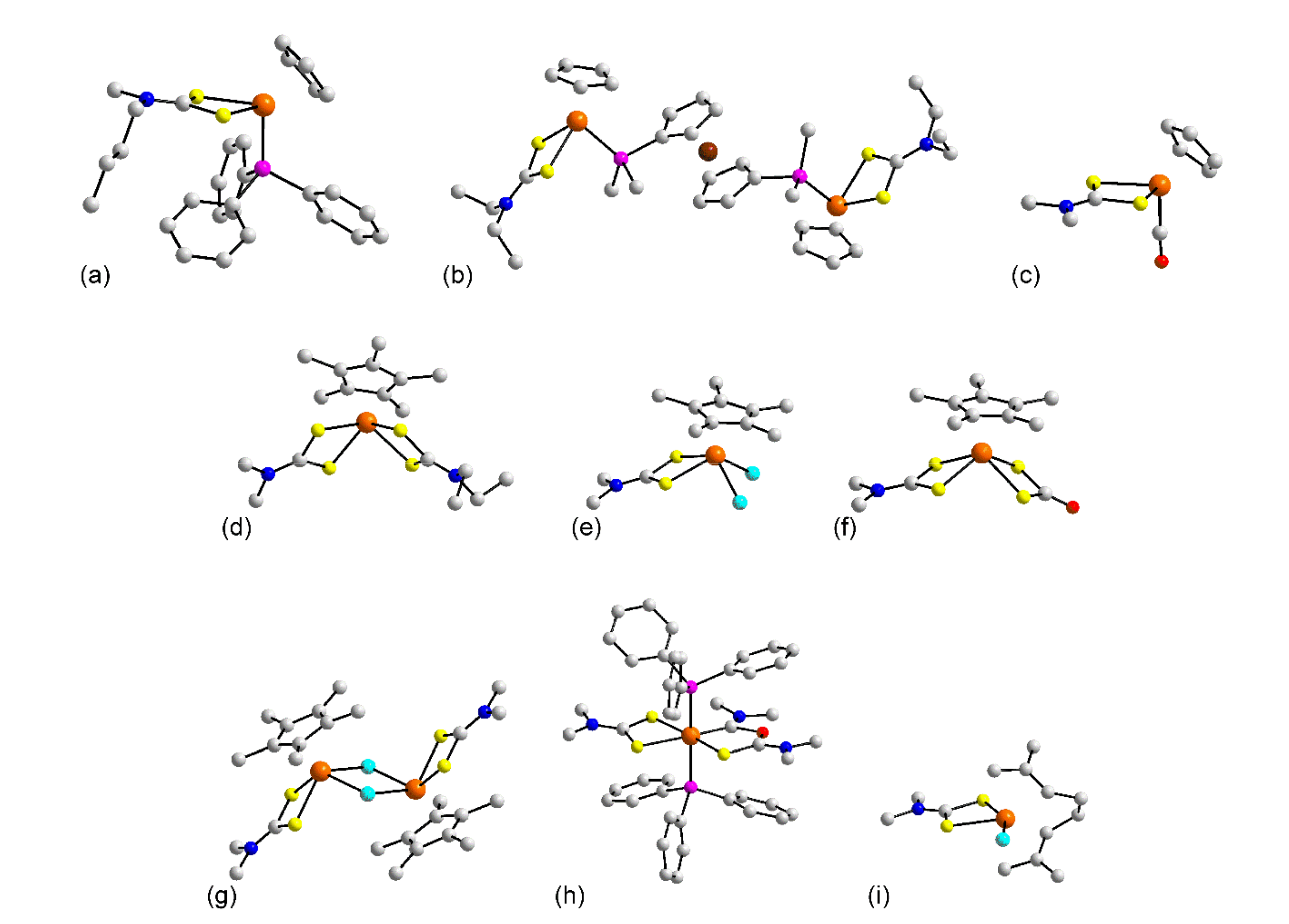
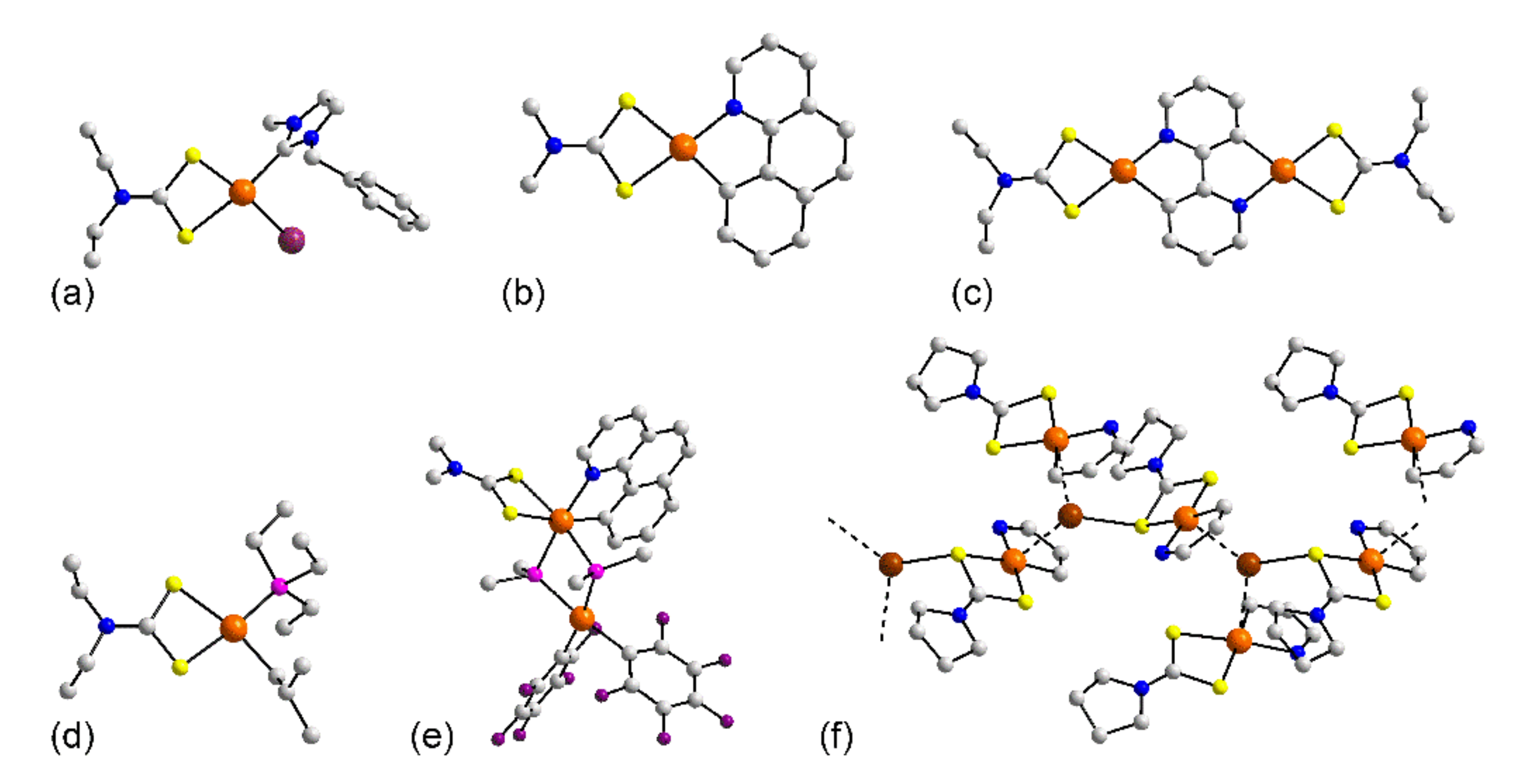
3.1. Solid-State Structures
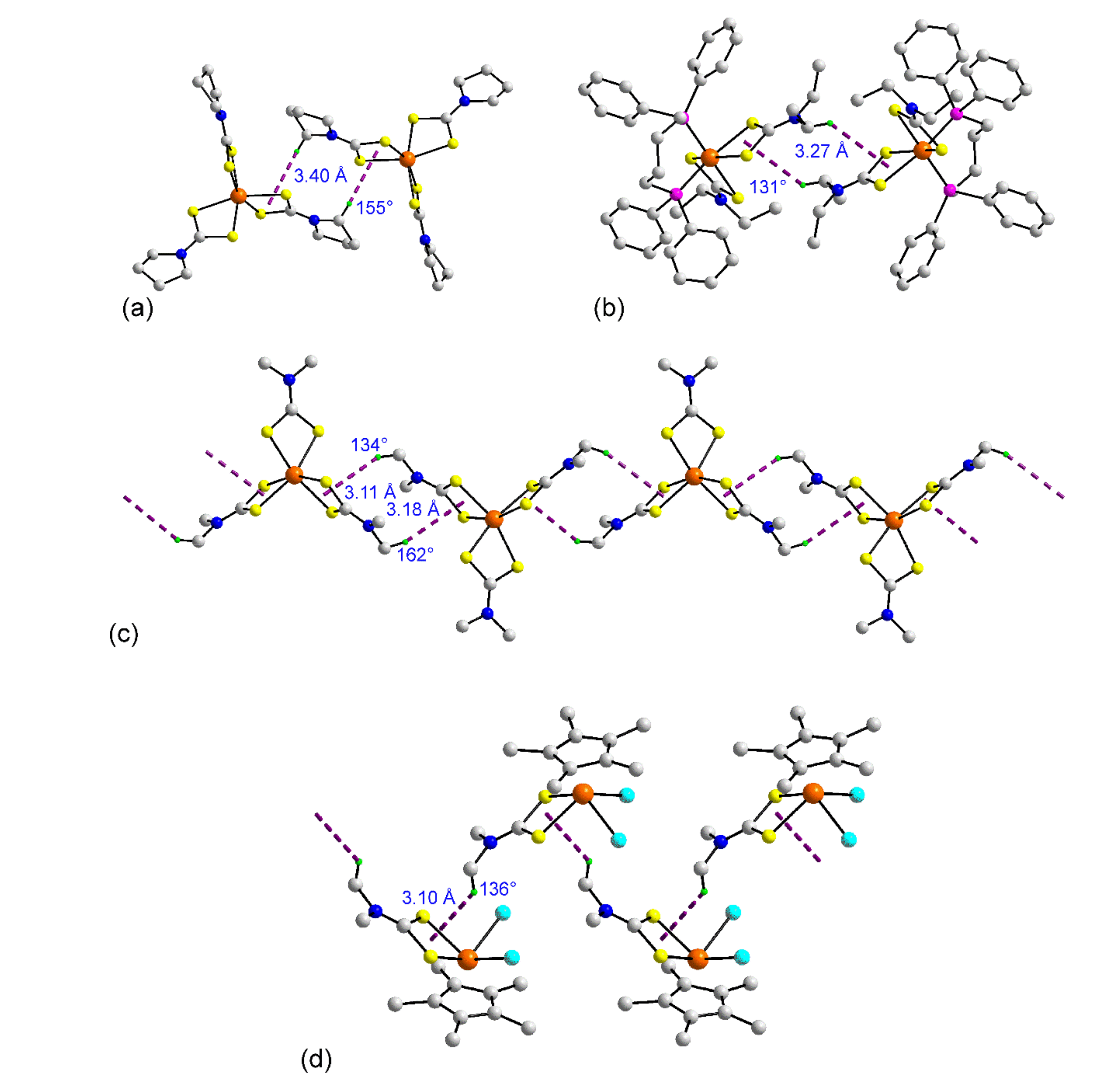
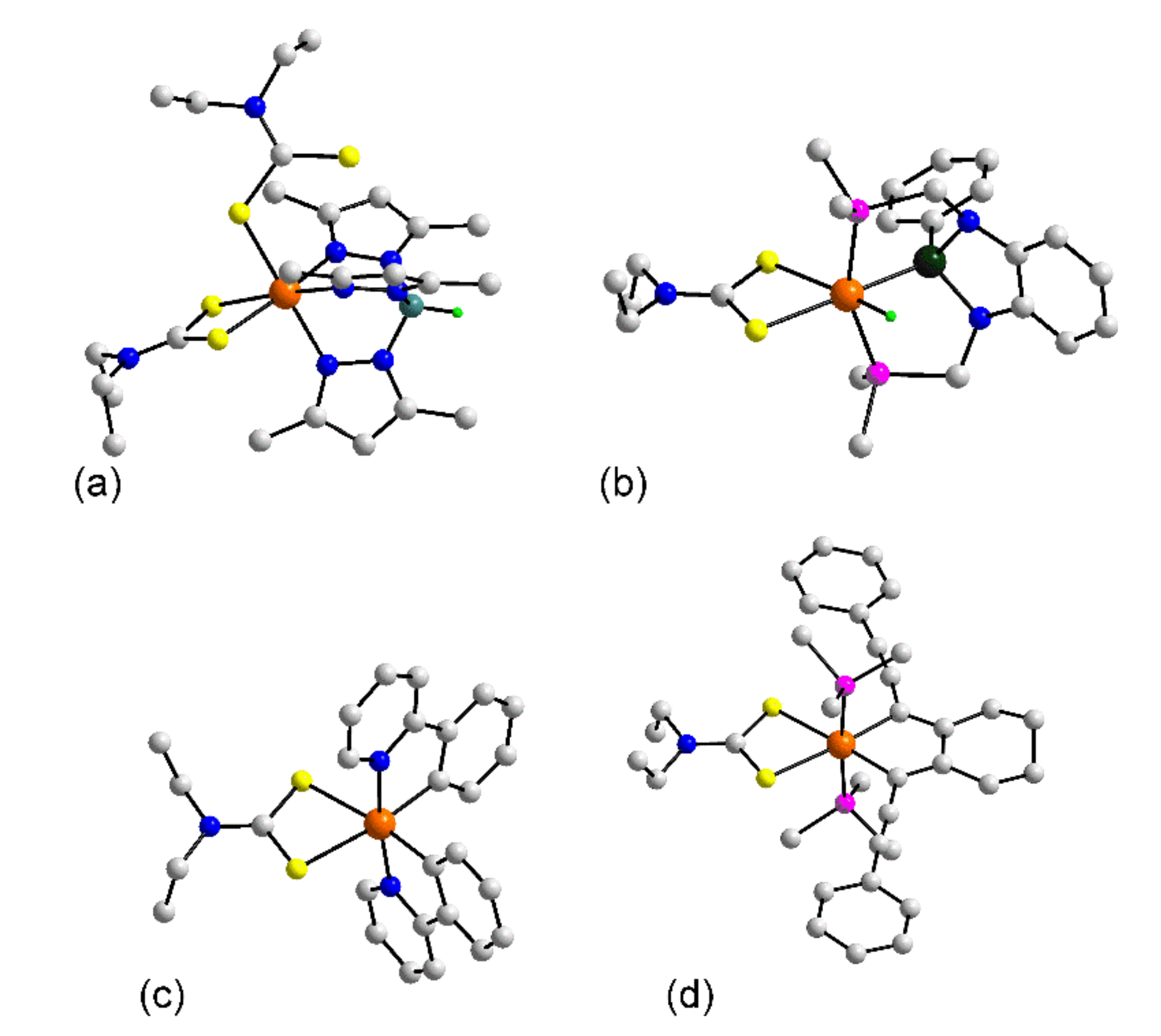
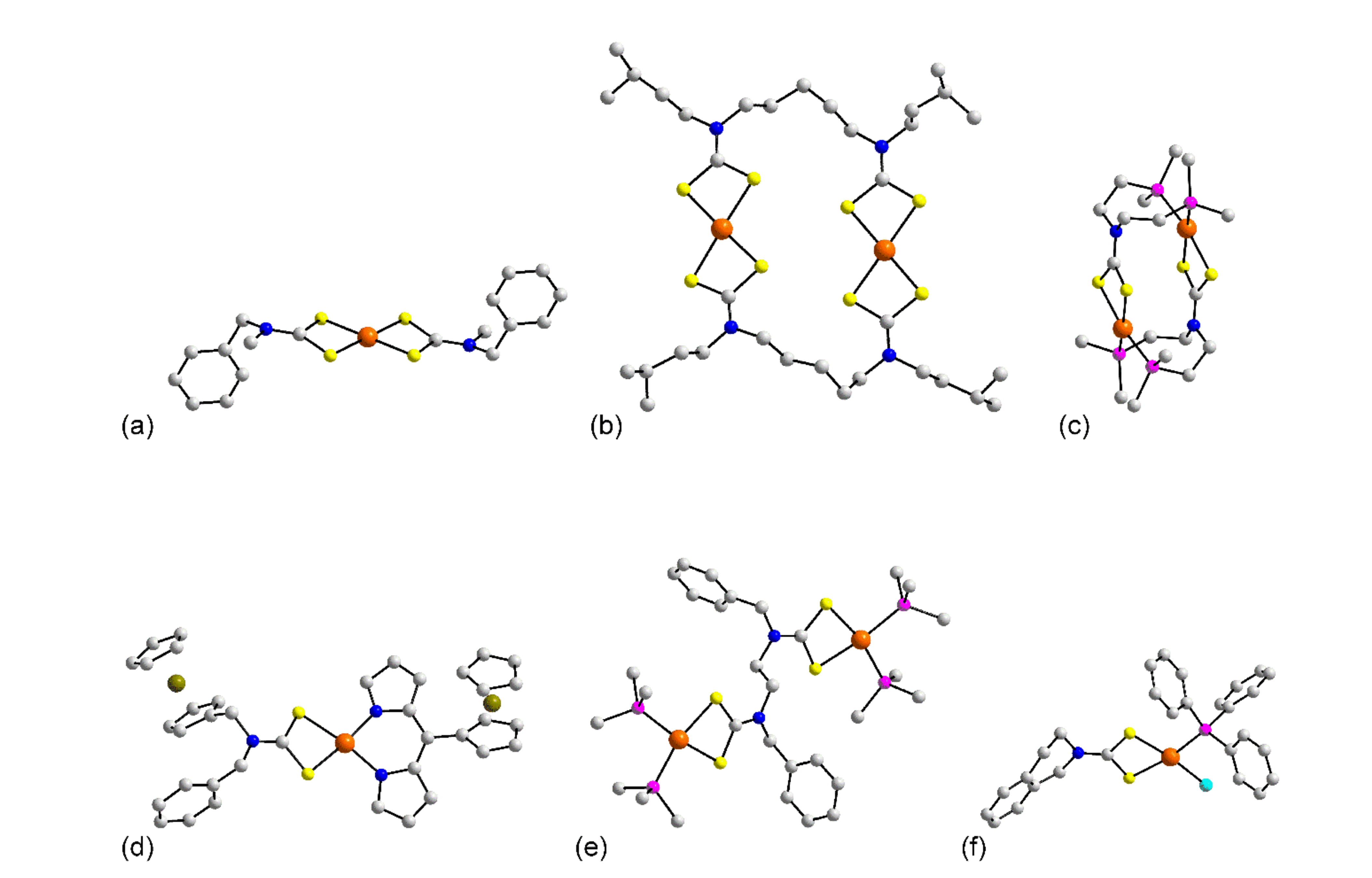
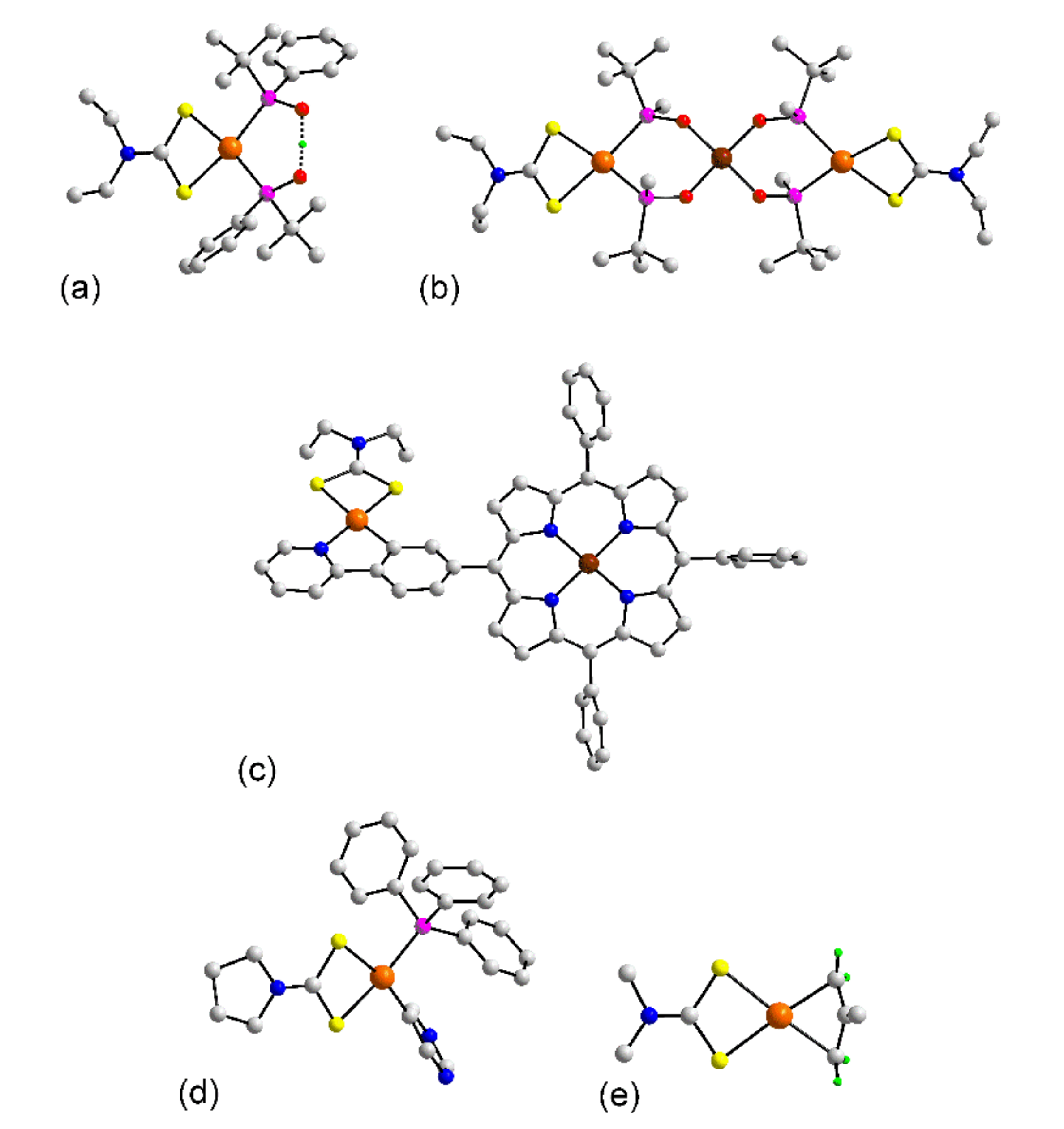
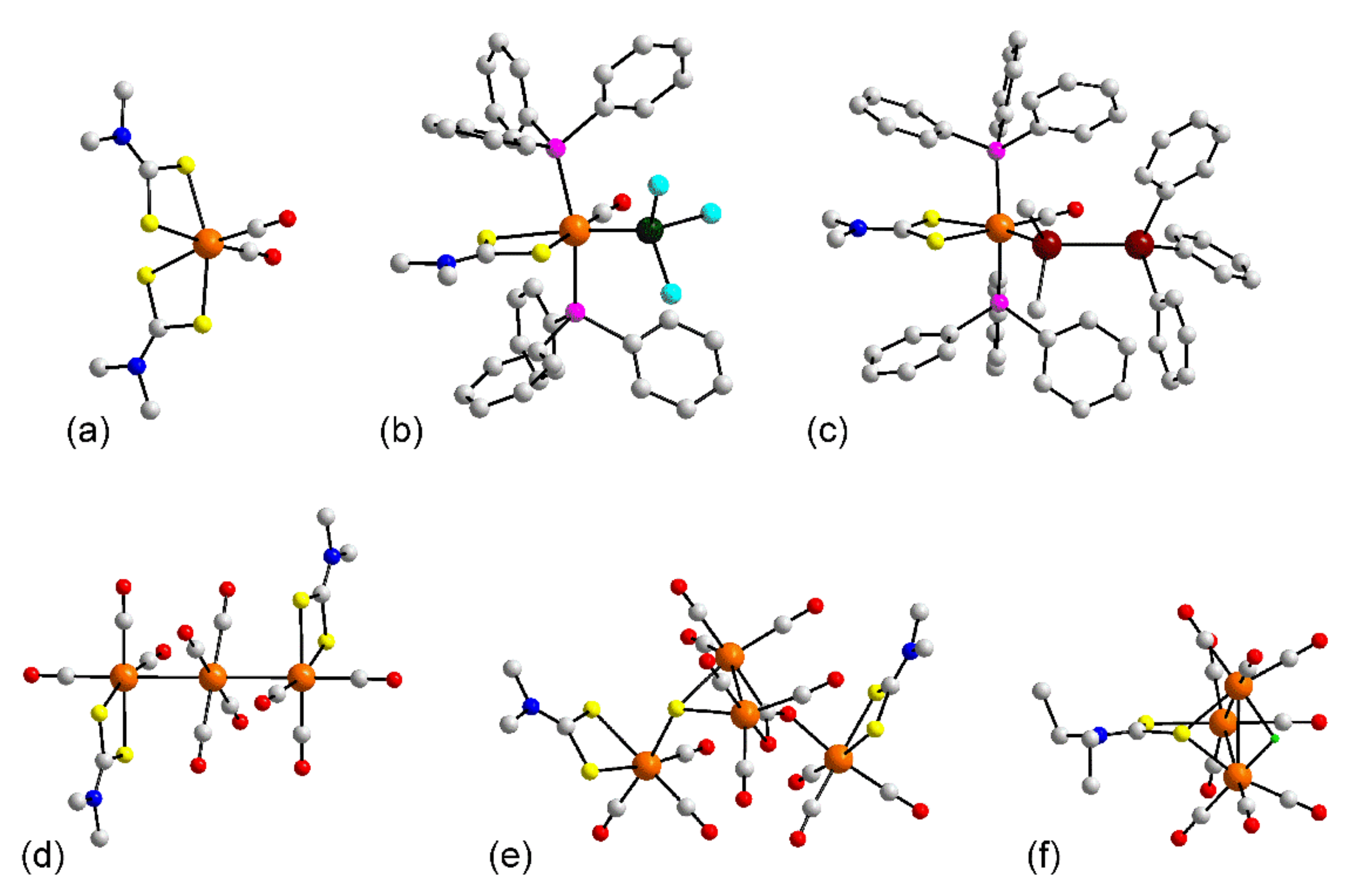
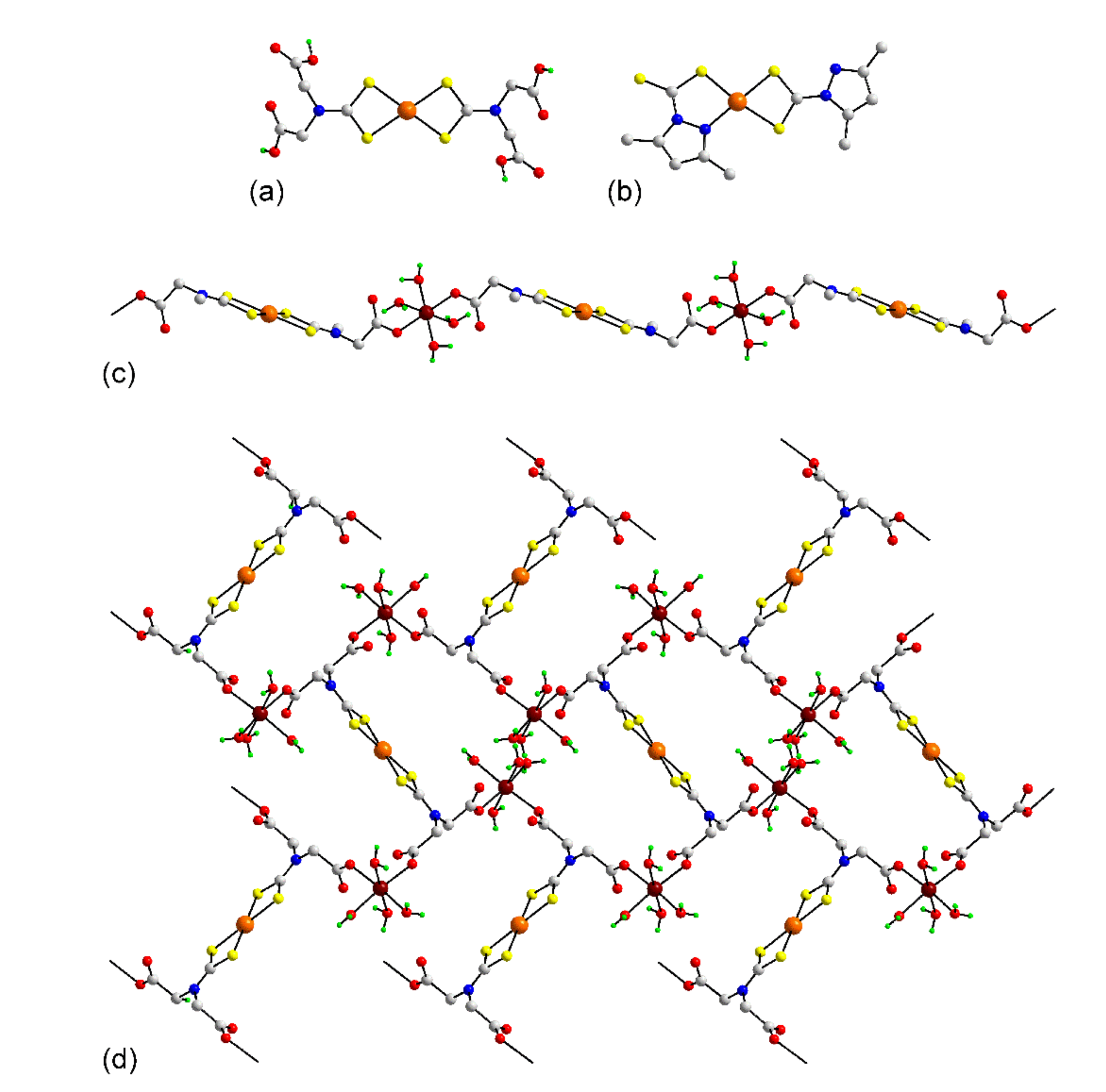
3.1.1. Ruthenium
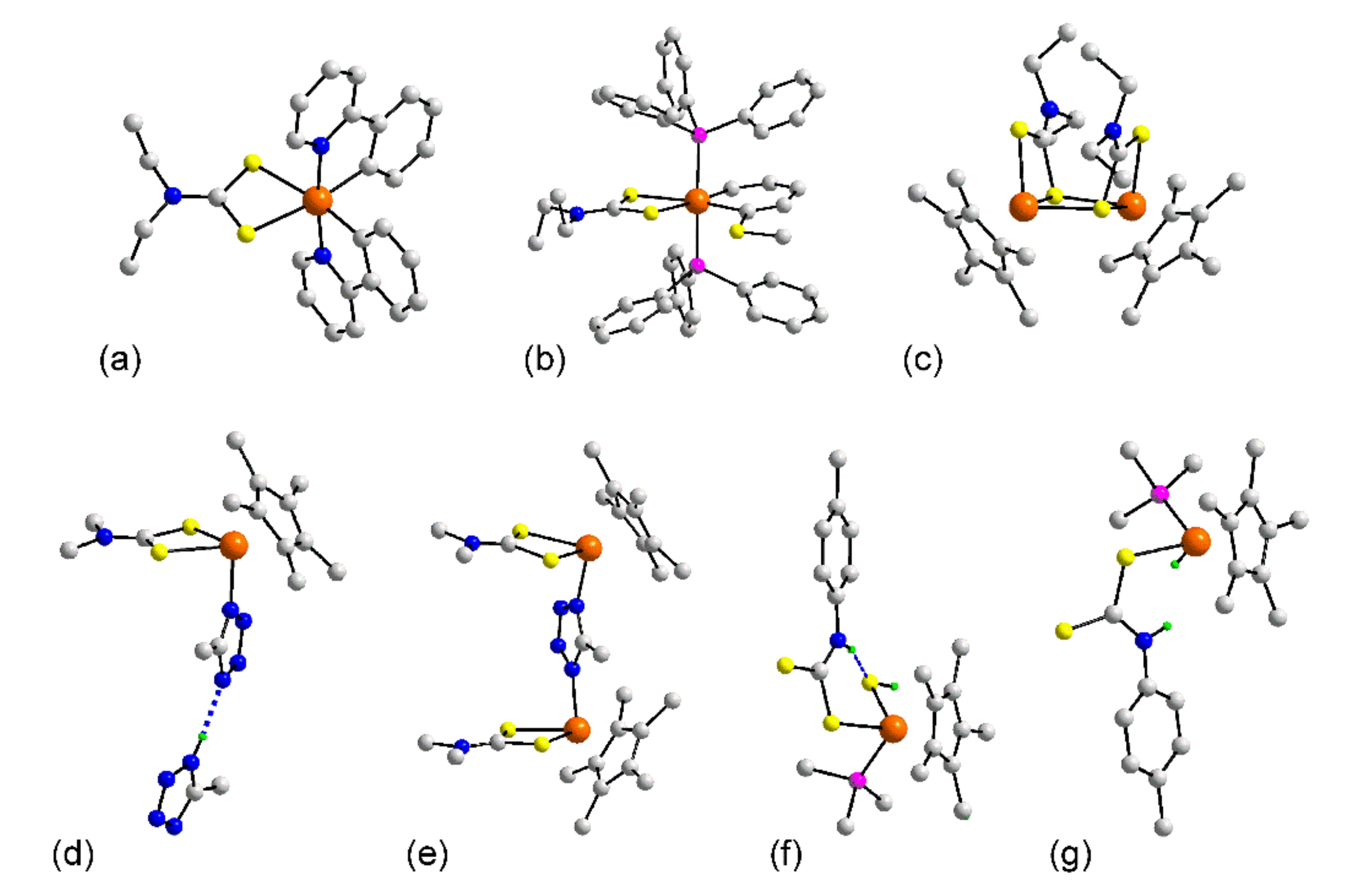
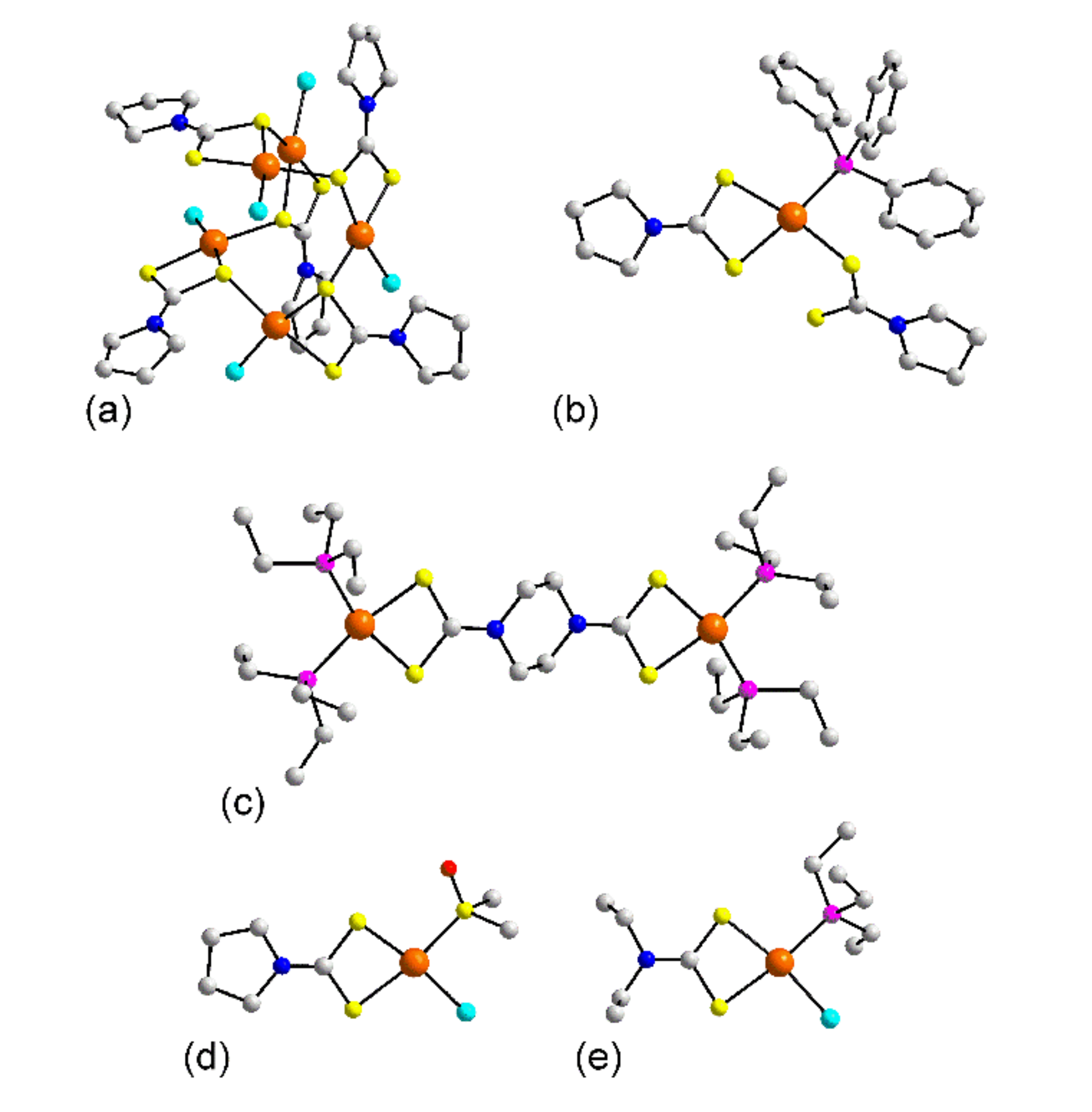
3.1.2. Rhodium
3.1.3. Palladium
3.1.4. Osmium
3.1.5. Iridium
3.1.6. Platinum
3.2. Nuclear Magnetic Resonance (NMR) Studies
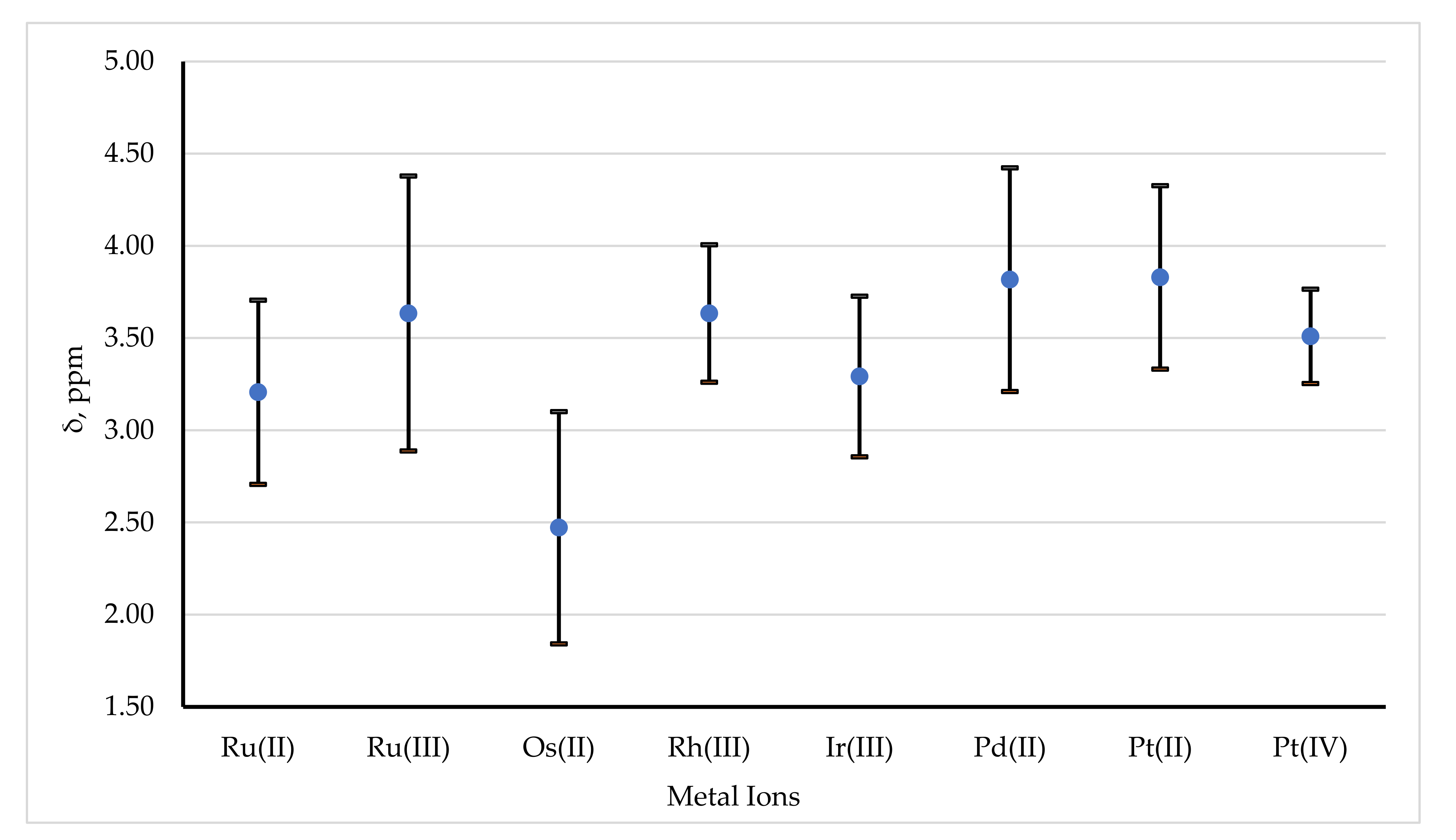
3.2.1. 1H NMR
- (i)
- Decrease down a Group,
- (ii)
- Increase across a Period,
- (iii)
- Increase with the oxidation state of the metal ion.
Barrier to C–N Bond Rotation
3.2.2. 13C NMR
3.2.3. 195Pt NMR
3.3. Applications
3.3.1. Biological Applications
| Complex a | IC50/μM b | Ref. | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LU | MCF7 | Heap-1c1c7 | PC-3 | MDA-MB-231 | HepG2/CTR | HepG2/SB3 | HeLa | HL60 | Daudi | LoVo | A549 | HTB-81 | MRC-5 | ||
| β-[Ru2(L57)5]Cl | - | - | - | - | - | 0.62(0.08) | 0.59(0.01) | 0.45(0.02) | - | - | - | - | - | - | [180] |
| β-[Ru2(L63)5]Cl | - | - | - | - | - | 0.66(0.01) | 0.75(0.02) | 0.46(0.04) | - | - | - | - | - | - | [180] |
| β-[Ru2(L60)5]Cl | - | - | - | - | - | 9.2(2) | >10 | 3.95(0.07) | - | - | - | - | - | - | [180] |
| β-[Ru2(L61)5]Cl | - | - | - | - | - | 1.28(0.05) | 0.90(0.02) | 0.27(0.01) | - | - | - | - | - | - | [180] |
| [PPh3Ru(η1-ClL)(CO)(L57)] | - | - | - | - | ≈25 | - | - | - | - | - | - | - | - | - | [56] |
| [PPh2(p-tolyl)Pd(L69)Cl] | 25.03(6.4) | 26.9(4.1) | 22.6(3.0) | 18.4(2.8) | 19.4(1.1) | - | - | - | - | - | - | - | - | - | [110] |
| 3.72 | 6.20 | 5.46 | [109] | ||||||||||||
| [P(p-tolyl)3Pd(L69)Cl] | 32.9(1.8) | 25.2(0.1) | 36.9(5.1) | 38.4(1.2) | 29.5(2.0) | - | - | - | - | - | - | - | - | - | [110] |
| 5.18 | 5.29 | 5.76 | [109] | ||||||||||||
| [PPh2(p-tolyl)Pd(L76)Cl] | 5.6(0.4) | 0.01(0.005) | 0.1(0.2) | 1.3(2.2) | 2.8(1.2) | - | - | - | - | - | - | - | - | - | [110] |
| [P(p-tolyl)3Pd(L76)Cl] | 2.9(0.1) | 2.21(3.5) | 12.2(1.9) | 15.0(3.9) | 7.1(1.9) | - | - | - | - | - | - | - | - | - | [110] |
| [PPh2(p-tolyl)Pd(L78)Cl] | 10.57(2.4) | 2.4(1.0) | 10(3) | 33.6(2.9) | 5.4(4.1) | - | - | - | - | - | - | - | - | - | [110] |
| [P(p-tolyl)3Pd(L78)Cl] | 10(2.4) | 3.02(0.3) | 2.8(0.4) | 3.9(0.024) | 7.0(3.6) | - | - | - | - | - | - | - | - | - | [110] |
| [PPh2(p-tolyl)Pd(L67)Cl] | - | 4.51 | - | 5.11 | - | - | - | - | - | - | - | 4.83 | - | - | [109] |
| [P(p-tolyl)3Pd(L67)Cl] | - | 4.12 | - | 7.12 | - | - | - | - | - | - | - | 7.21 | - | - | [109] |
| [PPh2(CH2Ph)Pd(L24)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | 3.67 | - | [114] |
| [PPh2(tert-But)Pd(L31)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | 9.52 | - | [115] |
| [PPh2(CH2Ph)Pd(L28)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | 4.57 | - | [115] |
| [PPh2(Cl)Pd(L28)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | 21.7 | - | [115] |
| [PPh3Pd(L26)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | 2.12 | - | [115] |
| [PPh2(tert-But)Pd(L38)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | 36.7(2.9) | [117] |
| [PPh2(nPr)Pd(L26)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | >100 | [117] |
| [PPh3Pd(L36)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | 71.5(13.1) | [117] |
| [PPh3Pd(L35)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | >100 | [117] |
| [PPh3Pd(L37)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | 81.6(13.1) | [117] |
| [PPh2(p-tolyl)Pd(L36)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | 22.8(2.4) | [117] |
| [PPh2(p-tolyl)Pd(L35)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | >100 | [117] |
| [PPh2(p-tolyl)Pd(L38)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | 30.8(2.6) | [117] |
| [PPh2(CH2Ph)Pd(L38)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | >100 | [117] |
| [PPh3Pd(L38)Cl] | - | - | - | - | - | - | - | - | - | - | - | - | - | 65.3(12.5) | [117] |
| [Pd(3-pic)(L20)Cl] | - | - | - | - | - | - | - | >100 | >100 | >100 | >100 | - | - | - | [198] |
| [Pd(2-pic)(L20)Cl] | - | - | - | - | - | - | - | 69.54(0.31) | 59.62(0.54) | 97.12(1.93) | 53.24(1.52) | - | - | - | [198] |
| [Pt(3-pic)(L20)Cl] | - | - | - | - | - | - | - | 98.90(2.33) | >100 | >100 | >100 | - | - | - | [198] |
| [Pt(2-pic)(L20)Cl] | - | - | - | - | - | - | - | 9.32(1.22) | 39.12(1.33) | 45.22(1.21) | 25.32(2.02) | - | - | - | [198] |
3.3.2. Other Applications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| bmi | 1-benzyl-3-methyl-imidazole |
| bpy | 2,2′-bipyridine |
| btfmppy | 2-(2,4-bis(trifluoromethyl)-phenyl)pyridine |
| buppy | 2-(4′-tert-butylphenyl)pyridine) |
| bzq | benzo(h)quinoline |
| C≡CBMIDA | C≡CB(O2CCH2)2NMe |
| cyclam | 1,4,8,11-tetraazacyclotetradecane |
| depe | 1,2-bis(diethylphosphino)ethane |
| dmbp | 4,4′-dimethyl-2,2′-bipyridine |
| dmso | dimethylsulfoxide |
| dpci | 3, 4-diphenylcinnoline |
| dpp | 4,6-diphenyl pyrimidine |
| dppb | 1,4-bis(diphenylphosphino)butane |
| dppe | 1,2-bis(diphenylphosphino)ethane |
| dppf | 1,1′-bis(diphenylphosphino)ferrocene |
| dppm | 1,1-bis(diphenylphosphino)methane |
| dppp | 1,3-bis(diphenylphosphino)propane |
| fcdpm | 5-Ferrocenyl dipyrromethene |
| mtz | 5-methyltetrazole |
| pba | 4-(2-pyridyl)benzaldehyde |
| pbt | 2-phenylbenzothiazole |
| p-cymene | 1-isopropyl-4-methylbenzene |
| phpy | 2-phenylpyridine |
| phpz | n-phenylpyrazole |
| pmea | 2-metallated 3-perylmethylen-4′-ethylaniline |
| ppy | 2-phenylpyridine |
| pqz | 4-phenylquinazoline |
| ptbt | 2-(p-tolyl)benzothiazole |
| pyr | pyrimidine |
| tfmbpy | 2′,6′-bis(trifluoromethyl)-2,4′-bipyridine |
| tfmpiq | 1-(4-(trifluoromethyl)phenyl)-isoquinoline |
| tfmppy | 2-(4-(trifluoromethyl)phenyl)pyridine |
| tfmpqz | 4-(4-(trifluoromethyl)phenyl)quinazoline |
| tmeda | N,N,N′,N′-Tetramethylethane-1,2-diamine |
| Tp* | HB(3,5-dimethylpyrazol-1-yl)3 |
| tptz | 2,4,6-tris(2-pyridyl)pyrazine-N,N′,N″ |
| X | 3-(((4-chlorophenyl)imino)methyl)-2-hydroxy-5-methylphenyl |
| X1 | η3, η3–2,7-dimethylocta-1,6-dien-3,8-diyl |
| X2 | 1,2-bis(2-phenylethynylmethylene)cyclohexane |
| X3 |  |
| X4 | 2,5-di-p-tolyl-1,3,4-oxadiazole |
| X5 | 3-perylenylmethylen-4′-ethylaniline |
References
- Coucouvanis, D. The chemistry of the dithioacid and 1,1-dithiolate complexes. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1970; Volume 11, pp. 234–371. [Google Scholar] [CrossRef]
- Eisenberg, R. Structural systematics of 1,1- and 1,2-dithiolato chelates. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1970; Volume 12, pp. 295–369. [Google Scholar] [CrossRef]
- Coucouvanis, D. The chemistry of the dithioacid and 1,1-dithiolate complexes, 1968–1977. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1979; Volume 26, pp. 301–469. [Google Scholar] [CrossRef]
- Hogarth, G. Transition metal dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 53, pp. 71–561. [Google Scholar] [CrossRef]
- Heard, P.J. Main group dithiocarbamate complexes. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 53, pp. 1–69. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Aggregation patterns in the crystal structures of organometallic Group XV 1,1-dithiolates: The influence of the Lewis acidity of the central atom, metal- and ligand-bound steric bulk, and coordination potential of the 1,1-dithiolate ligands upon supramolecular architecture. CrystEngComm 2006, 8, 104–118. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Stereochemical activity of lone pairs of electrons and supramolecular aggregation patterns based on secondary interactions involving tellurium in its 1,1-dithiolate structures. Coord. Chem. Rev. 2010, 254, 46–76. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Exploring the topological landscape exhibited by binary zinc-triad 1,1-dithiolates. Crystals 2018, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Tiekink, E.R.T. Perplexing coordination behaviour of potentially bridging bipyridyl-type ligands in the coordination chemistry of zinc and cadmium 1,1-dithiolate compounds. Crystals 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Tiekink, E.R.T. A structural survey of poly-functional dithiocarbamate ligands and the aggregation patterns they sustain. Inorganics 2021, 9, 7. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. On the coordination role of pyridyl-nitrogen in the structural chemistry of pyridyl-substituted dithiocarbamate ligands. Crystals 2021, 11, 286. [Google Scholar] [CrossRef]
- Masui, H. Metalloaromaticity. Coord. Chem. Rev. 2001, 219–221, 957–992. [Google Scholar] [CrossRef]
- Tiekink, E.R.T.; Zukerman-Schpector, J. Emerging supramolecular synthons: C–H⋯π(chelate) interactions in metal bis(1,1-dithiolates). Chem. Commun. 2011, 47, 6623–6625. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. The remarkable propensity for the formation of C–H⋯π(chelate ring) interactions in the crystals of the first-row transition metal dithiocarbamates and the supramolecular architectures they sustain. CrystEngComm 2020, 22, 7308–7733. [Google Scholar] [CrossRef]
- Sredojević, D.; Bogdanović, G.A.; Tomić, Z.D.; Zarić, S.D. Stacking vs. CH–π interactions between chelate and aryl rings in crystal structures of square-planar transition metal complexes. CrystEngComm 2007, 9, 793–798. [Google Scholar] [CrossRef]
- Tan, Y.S.; Halim, S.N.A.; Molloy, K.C.; Sudlow, A.L.; Otero-de-la-Roza, A.; Tiekink, E.R.T. Persistence of C–H⋯π(chelate ring) interactions in the crystal structures of Pd(S2COR)2. The utility of Pd(S2COR)2 as precursors for palladium sulphide materials. CrystEngComm 2016, 18, 1105–1117. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.D.; Sakla, A.P.; Shankaraiah, N. An insight into medicinal attributes of dithiocarbamates: Bird’s eye view. Bioorg. Chem. 2020, 105, 104346. [Google Scholar] [CrossRef] [PubMed]
- Buac, D.; Schmitt, S.; Ventro, G.; Kona, F.R.; Dou, Q.P. Dithiocarbamate-based coordination compounds as potent proteasome inhibitors in human cancer cells. Mini Rev. Med. Chem. 2012, 12, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Nagy, E.M.; Ronconi, L.; Nardon, C.; Fregona, D. Noble metal-dithiocarbamates precious allies in the fight against cancer. Mini Rev. Med. Chem. 2012, 12, 1216–1229. [Google Scholar] [CrossRef]
- Hogarth, G. Metal-dithiocarbamate complexes: Chemistry and biological activity. Mini Rev. Med. Chem. 2012, 12, 1202–1215. [Google Scholar] [CrossRef]
- Ahmed, A.J. Metal complexes of dithiocarbamate derivatives and its biological activity. Asian J. Chem. 2018, 30, 2595–2602. [Google Scholar] [CrossRef]
- Sarker, J.C.; Hogarth, G. Dithiocarbamate complexes as single source precursors to nanoscale binary, ternary and quaternary metal sulfides. Chem. Rev. 2021, 121, 6057–6123. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.; Haigh, S.; O’Brien, P. The synthesis of metallic and semiconducting nanoparticles from reactive melts of precursors. J. Mater. Chem. A 2014, 2, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Nagy, M.; Nardon, C.; Giovagnini, L.; Marchiò, L.; Trevisan, A.; Fregona, D. Promising anticancer mono- and dinuclear ruthenium(III) dithiocarbamato complexes: Systematic solution studies. Dalton Trans. 2011, 40, 11885–11895. [Google Scholar] [CrossRef] [PubMed]
- Almazahreh, L.; El-khateeb, M.; Harb, M.; Görls, H.; Weigand, W. Synthesis and characterization of platinum and palladium pyrrolidinedithiocarbamate complexes. Transit. Met. Chem. 2013, 38, 377–383. [Google Scholar] [CrossRef]
- Mei, Q.; Shi, Y.; Hua, Q.; Tong, B. Phosphorescent chemosensor for Hg2+ based on an iridium(iii) complex coordinated with 4-phenylquinazoline and carbazole dithiocarbamate. RSC Adv. 2015, 5, 74924–74931. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Churakov, A.V.; Howard, J.A.K.; Hughes, A.K.; Johnson, A.L.; Kingsley, A.J.; Neretin, I.S.; Wade, K. Transition metal dicarbollide complexes: Synthesis, molecular, crystal and electronic structures of [M(C2B9H11)(NMe2)3] (M = Nb or Ta) and their insertion reactions with CO2 and CS2. J. Chem. Soc. Dalton Trans. 1999, 3867–3875. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A million crystal structures: The whole is greater than the sum of its parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Brandenburg, K.; Berndt, M. DIAMOND, Version 3.2k; GbR: Bonn, Germany, 2006. [Google Scholar]
- Nagy, E.M.; Pettenuzzo, A.; Boscutti, G.; Marchi, L.; Via, L.D.; Fregona, D. Ruthenium(II/III)-based compounds with encouraging antiproliferative activity against non-small-cell lung cancer. Chem. Eur. J. 2012, 18, 14464–14472. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-H.; Liu, Y.-L.; Duan, T.; Lu, L.; Zhang, Q.-F.; Leung, W.-H. Synthesis, structure and electrochemistry of [Ru2(S2CNMe2)3(µ3-S2CNMe2)2]2[Mo6O19]·2CH3COCH3. Z. Naturforsch. B Chem. Sci. 2009, 64, 800–804. [Google Scholar] [CrossRef]
- Santos, K.; Dinelli, L.R.; Bogado, A.L.; Ramos, L.A.; Cavalheiro, É.T.; Ellena, J.; Castellano, E.E.; Batista, A.A. Crystal structure and catalytic activity of ruthenium (II)/dithiocarbamate complexes in the epoxidation of cyclooctene. Inorg. Chim. Acta 2015, 429, 237–242. [Google Scholar] [CrossRef]
- Pinheiro, S.O.; de Sousa, J.R.; Santiago, M.O.; Carvalho, I.M.M.; Silva, A.L.R.; Batista, A.A.; Castellano, E.E.; Ellena, J.; Moreira, I.S.; Diogenes, I.C.N. Synthesis, characterization and structure of ruthenium(II) phosphine complexes with N-heterocyclic thiolate ligands. Inorg. Chim. Acta 2006, 359, 391–400. [Google Scholar] [CrossRef]
- Ng, S.; Ziller, J.W.; Farmer, P.J. Multiple pathways for the oxygenation of a ruthenium(II) dithiocarbamate complex: S-oxygenation and S-extrusion. Inorg. Chem. 2004, 43, 8301–8309. [Google Scholar] [CrossRef] [PubMed]
- Cookson, J.; Vickers, M.S.; Paul, R.L.; Cowley, A.R.; Beer, P.D. Amide functionalised dithiocarbamate ruthenium(II) bis-bipyridyl receptors: A new class of redox-responsive anion sensor. Inorg. Chim. Acta 2008, 361, 1689–1698. [Google Scholar] [CrossRef]
- Milsmann, C.; Bill, E.; Weyhermuller, T.; George, S.D.; Wieghardt, K. Electronic structures of [RuII(cyclam)(Et2dtc)]+, [Ru(cyclam)(tdt)]+, and [Ru(cyclam)(tdt)]2+: An X-ray absorption spectroscopic and computational study (tdt = toluene-3,4-dithiolate; Et2dtc = N,N-diethyldithiocarbamate(1-)). Inorg. Chem. 2009, 48, 9754–9766. [Google Scholar] [CrossRef] [PubMed]
- Hurtubise, V.L.; McArdle, J.M.; Naeem, S.; Toscani, A.; White, A.J.P.; Long, N.J.; Wilton-Ely, J.D.E.T. Multimetallic complexes and functionalized nanoparticles based on unsymmetrical dithiocarbamate ligands with allyl and propargyl functionality. Inorg. Chem. 2014, 53, 11740–11748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeem, S.; Ogilvie, E.; White, A.J.P.; Hogarth, G.; Wilton-Ely, J.D.E.T. The functionalisation of ruthenium(II) and osmium(II) alkenyl complexes with amine- and alkoxy-terminated dithiocarbamates. Dalton Trans. 2010, 39, 4080–4089. [Google Scholar] [CrossRef] [PubMed]
- Wilton-Ely, J.D.E.T.; Solanki, D.; Hogarth, G. Multifunctional dithiocarbamates as ligands towards the rational synthesis of polymetallic arrays: An example based on a piperizine-derived dithiocarbamate ligand. Eur. J. Inorg. Chem. 2005, 2005, 4027–4030. [Google Scholar] [CrossRef]
- Knight, E.J.; Cowley, A.R.; Hogarth, G.; Wilton-Ely, J.D.E.T. Bifunctional dithiocarbamates: A bridge between coordination chemistry and nanoscale materials. Dalton Trans. 2009, 607–609. [Google Scholar] [CrossRef]
- Wu, F.-H.; Duan, T.; Lu, L.; Zhang, Q.-F.; Leung, W.-H. Synthesis and reactivity of ruthenium complexes with 1,1′-dithiolate ligands. J. Organomet. Chem. 2009, 694, 3844–3851. [Google Scholar] [CrossRef]
- Wu, F.-H.; Duan, T.-K.; Lu, L.-D.; Zhang, Q.-F. Syntheses and structures of two ruthenium(III) complexes [Ru(PPh3)2(S2CNR2)Cl2]·CH2Cl2(R=Et and iPr). Chin. J. Struct. Chem. 2010, 29, 23–28. [Google Scholar]
- Sharma, S.; Chandra, M.; Pandey, D.S. New multifunctional complexes [Ru(κ3-L)(EPh3)2Cl]+ [E = P, As; L = 2,4,6-Tris(2-pyridyl)-1,3,5-triazine] containing both group V and polypyridyl ligands. Eur. J. Inorg. Chem. 2004, 2004, 3555–3563. [Google Scholar] [CrossRef]
- Deeming, A.J.; Forth, C.; Hogarth, G. Reactions of trans-[RuCl2(CO)2(PEt3)2] with 1,1-dithiolates: Stepwise formation of cis-[Ru(CO)(PEt3)(S2X)] (X = CNMe2, CNEt2, COEt, P(OEt)2, PPh2). J. Organomet. Chem. 2006, 691, 79–85. [Google Scholar] [CrossRef]
- Boyd, P.D.W.; Hart, M.C.; Pritzwald-Stegmann, J.R.F.; Roper, W.R.; Wright, L.J. Selective substitution of one of the substituents on germanium in coordinatively unsaturated ruthenium germyl complexes. Organometallics 2012, 31, 2914–2921. [Google Scholar] [CrossRef]
- Kwok, W.-H.; Lu, G.-L.; Rickard, C.E.F.; Roper, W.R.; Wright, L.J. Tethered silyl complexes from nucleophilic substitution reactions at the Si–Cl bond of the chloro(diphenyl)silyl ligand in Ru(SiClPh2)(κ2-S2CNMe2)(CO)(PPh3)2. J. Organomet. Chem. 2004, 689, 2979–2987. [Google Scholar] [CrossRef]
- McQueen, C.M.A.; Hill, A.F.; Sharma, M.; Singh, S.K.; Ward, J.S.; Willis, A.C.; Young, R.D. Synthesis and reactivity of osmium and ruthenium PBP–LXL boryl pincer complexes. Polyhedron 2016, 120, 185–195. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Shi, H.-T.; Zhang, Q.-F. Synthesis and structure of a ruthenium(II)-dithiocarbamate complex [RuH(CO)(PPh3)2(4-CIPhNHCS2)]·C6H14. Chin. J. Struct. Chem. 2011, 30, 470–473. [Google Scholar]
- Toscani, A.; Heliövaara, E.K.; Hena, J.B.; White, A.J.P.; Wilton-Ely, J.D.E.T. Multimetallic alkenyl complexes bearing macrocyclic dithiocarbamate ligands. Organometallics 2015, 34, 494–505. [Google Scholar] [CrossRef]
- Hill, A.F.; Stewart, C.D.; Ward, J.S. Organometallic chemistry of ethynyl boronic acid MIDA ester, HC [triple bond, length as m-dash] CB (O 2 CCH 2) 2 NMe. Dalton Trans. 2015, 44, 5713–5726. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S.; White, A.J.P.; Hogarth, G.; Wilton-Ely, J.D.E.T. Multifunctional dithiocarbamates: Synthesis and ring-closing metathesis of diallyldithiocarbamate complexes. Organometallics 2010, 29, 2547–2556. [Google Scholar] [CrossRef]
- Bartlett, M.J.; Frogley, B.J.; Hill, A.F.; Sharma, M.; Smith, M.K.; Ward, J.S. Hydrogenating an organometallic carbon chain: Buten-yn-diyl (CH=CHC≡C) as a missing link. Dalton Trans. 2019, 48, 16534–16554. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, M.J.; Hogarth, G.; Thompson, A.L.; Wilton-Ely, J.D.E.T. Multimetallic arrays: Symmetrical and unsymmetrical bi-, tri-, and tetrametallic organometallic complexes of ruthenium(II) and osmium(II). Organometallics 2009, 28, 197–208. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mandal, S.; Mohapatra, S.; Chatterjee, A.; Bhattacharyya, A.; Chattopadhyay, S. Spectroscopic, structural, electrochemical, and cytotoxicity studies on dithiocarbamato-chelated ruthenium organometallics incorporating imine-phenol function. J. Coord. Chem. 2019, 72, 180–200. [Google Scholar] [CrossRef]
- Ollivier, A.; Foresti, R.; El Ali, Z.; Martens, T.; Kitagishi, H.; Motterlini, R.; Rivard, M. Design and biological evaluation of manganese- and ruthenium-based hybrid CO-RMs (HYCOs). ChemMedChem 2019, 14, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, G.; Faulkner, S. Cyclopentadienyl ruthenium(II) dithiocarbamate complexes: Crystal structures of [CpRu(PPh3)(S2CNPr2)] and [CpRu(PPh3)(S2CNMeBu)]. Inorg. Chim. Acta 2006, 359, 1018–1022. [Google Scholar] [CrossRef]
- Lalrempuia, R.; Suante, H.; Yennawar, H.P.; Kollipara, M.R. Reactivity of the [CpM(PPh3)2]+ (M=Ru, Os) fragment with diethyldithiocarbamate. Polyhedron 2007, 26, 867–870. [Google Scholar] [CrossRef]
- El-Khateeb, M.; Al-Noaimi, M.; Harb, M.; Gorls, H.; Weigand, W. Half-sandwich ruthenium complexes of heterocyclic-dithiocarboxylato ligands. Jordan J. Chem. 2008, 3, 147. [Google Scholar]
- Lu, X.L.; Vittal, J.J.; Tiekink, E.R.T.; Tan, G.K.; Kuan, S.L.; Goh, L.Y.; Hor, T.S.A. Comparative reactivity studies of dppf-containing CpRuII and (C6Me6)RuII complexes towards different donor ligands (dppf=1,1′-bis(diphenylphosphino)ferrocene). J. Organomet. Chem. 2004, 689, 1978–1990. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Bitterwolf, T.E. Carbonyl-cyclopentadienyl-(N,N-dimethyldithiocarbamato)-ruthenium. CSD Commun. 2019. [Google Scholar] [CrossRef]
- Cheung, W.-M.; Zhang, Q.-F.; Wiliams, I.D.; Leung, W.-H. An unsaturated half-sandwich ruthenium(II) complex containing a dithioimidodiphosphinate ligand. Inorg. Chim. Acta 2006, 359, 782–788. [Google Scholar] [CrossRef]
- Tay, E.P.L.; Kuan, S.L.; Leong, W.K.; Goh, L.Y. Synthetic and X-ray structural and reactivity studies of Cp*RuIV complexes containing bidentate dithiocarbonate, xanthate, carbonate, and phosphinate ligands (Cp* = η5-C5Me5). Inorg. Chem. 2007, 46, 1440–1450. [Google Scholar] [CrossRef]
- Kuan, S.L.; Tay, E.P.L.; Leong, W.K.; Goh, L.Y.; Lin, C.Y.; Gill, P.M.W.; Webster, R.D. Highly oxidized ruthenium organometallic compounds. The synthesis and one-electron electrochemical oxidation of [Cp*RuIVCl2(S2CR)] (Cp* = η5-C5Me5, R = NMe2, NEt2, OiPr). Organometallics 2006, 25, 6134–6141. [Google Scholar] [CrossRef]
- Xu, C.; Pullarkat, S.A.; Goh, L.Y. Bis(allyl)ruthenium(iv)-initiated S–S and C–S bond cleavages in tetraalkylthiuram sulfides. Formation and X-ray crystal structures of dithiocarbamato complexes. Aust. J. Chem. 2009, 62, 1537–1543. [Google Scholar] [CrossRef]
- Dou, Y.-H.; Xu, S.-D.; Chen, Y.; Wu, X.-H. Synthesis, characterization, and anticancer activity of dithiocarbamate ruthenium(II) complexes. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 1219–1223. [Google Scholar] [CrossRef]
- Seino, H.; Yoshikawa, T.; Hidai, M.; Mizobe, Y. Preparation of mononuclear and dinuclear Rh hydrotris(pyrazolyl)borato complexes containing arenethiolato ligands and conversion of the mononuclear complexes into dinuclear Rh–Rh and Rh–Ir complexes with bridging arenethiolato ligands. Dalton Trans. 2004, 3593–3600. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.S.H.; Hill, A.F.; Sinha, A.; Ward, J.S. N-heterocyclic silyl pincer ligands. Organometallics 2014, 33, 653–658. [Google Scholar] [CrossRef]
- Mansouri, G.; Heidarizadi, F.; Naghipour, A.; Notash, B. Synthesis, characterization and antibacterial study of cyclometalated rhodium(III) complex containing dithiocarbamate. J. Mol. Struct. 2016, 1121, 128–134. [Google Scholar] [CrossRef]
- Mukherjee, T.; Sen, B.; Patra, A.; Banerjee, S.; Hundal, G.; Chattopadhyay, P. Cyclometalated rhodium(III) complexes bearing dithiocarbamate derivative: Synthesis, characterization, interaction with DNA and biological study. Polyhedron 2014, 69, 127–134. [Google Scholar] [CrossRef]
- Steffen, A.; Tay, M.G.; Batsanov, A.S.; Howard, J.A.K.; Beeby, A.; Vuong, K.Q.; Sun, X.-Z.; George, M.W.; Marder, T.B. 2,5-Bis(p-R-arylethynyl)rhodacyclopentadienes show intense fluorescence: Denying the presence of a heavy atom. Angew. Chem. Int. Ed. 2010, 49, 2349–2353. [Google Scholar] [CrossRef] [PubMed]
- Bobinihi, F.F.; Onwudiwe, D.C.; Ekennia, A.C.; Okpareke, O.C.; Arderne, C.; Lane, J.R. Group 10 metal complexes of dithiocarbamates derived from primary anilines: Synthesis, characterization, computational and antimicrobial studies. Polyhedron 2019, 158, 296–310. [Google Scholar] [CrossRef]
- Poirier, S.; Tailleur, E.; Lynn, H.; Reber, C. Characterization of Pd⋯H–C interactions in bis-dimethyldithiocarbamate palladium(II) and its deuterated analog by luminescence spectroscopy at variable pressure. Dalton Trans. 2016, 45, 10883–10886. [Google Scholar] [CrossRef]
- Poirier, S.; Lynn, H.; Reber, C.; Tailleur, E.; Marchivie, M.; Guionneau, P.; Probert, M.R. Variation of M···H–C interactions in square-planar complexes of nickel(II), palladium(II), and platinum(II) probed by luminescence spectroscopy and X-ray diffraction at variable pressure. Inorg. Chem. 2018, 57, 7713–7723. [Google Scholar] [CrossRef] [Green Version]
- Poirier, S.; Guionneau, P.; Luneau, D.; Reber, C. Why do the luminescence maxima of isostructural palladium(II) and platinum(II) complexes shift in opposite directions? Can. J. Chem. 2014, 92, 958–965. [Google Scholar] [CrossRef] [Green Version]
- Birri, A.; Harvey, B.; Hogarth, G.; Subasi, E.; Ugur, F. Allyl palladium dithiocarbamates and related dithiolate complexes as precursors to palladium sulfides. J. Organomet. Chem. 2007, 692, 2448–2455. [Google Scholar] [CrossRef]
- Shaheen, F.; Badshah, A.; Gielen, M.; Dusek, M.; Fejfarova, K.; de Vos, D.; Mirza, B. Synthesis, characterization, antibacterial and cytotoxic activity of new palladium(II) complexes with dithiocarbamate ligands: X-ray structure of bis(dibenzyl-1-S:S′-dithiocarbamato)Pd(II). J. Organomet. Chem. 2007, 692, 3019–3026. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Ming, H.N.; McKee, V.; Peiris, T.A.N.; Wijayantha-Kahagala-Gamage, U.; Arifin, Z.; Mazhar, M. Vysotskite structured photoactive palladium sulphide thin films from dithiocarbamate derivatives. New J. Chem. 2014, 38, 4083–4091. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.P.; de Lima, G.M.; Paniago, E.B.; Takahashi, J.A.; Pinheiro, C.B. Synthesis, characterization and antifungal activity of new dithiocarbamate-based complexes of Ni(II), Pd(II) and Pt(II). Inorg. Chim. Acta 2014, 423, 443–449. [Google Scholar] [CrossRef]
- Phadnis, P.P.; Jain, V.K.; Schurr, T.; Klein, A.; Lissner, F.; Schleid, T.; Kaim, W. Synthesis, spectroscopy, structure and photophysical properties of dinaphthylmethylarsine complexes of palladium(II) and platinum(II). Inorg. Chim. Acta 2005, 358, 2609–2617. [Google Scholar] [CrossRef]
- Bobinihi, F.F.; Onwudiwe, D.C.; Hosten, E.C. Synthesis and characterization of homoleptic group 10 dithiocarbamate complexes and heteroleptic Ni(II) complexes, and the use of the homoleptic Ni(II) for the preparation of nickel sulphide nanoparticles. J. Mol. Struct. 2018, 1164, 475–485. [Google Scholar] [CrossRef]
- Hogarth, G.; Rainford-Brent, E.-J.C.-R.C.R.; Kabir, S.E.; Richards, I.; Wilton-Ely, J.D.E.T.; Zhang, Q. Functionalised dithiocarbamate complexes: Synthesis and molecular structures of 2-diethylaminoethyl and 3-dimethylaminopropyl dithiocarbamate complexes [M{S2CN(CH2CH2NEt2)2}n] and [M{S2CN(CH2CH2CH2NMe2)2}n] (n=2, M=Ni, Cu, Zn, Pd; n=3, M=Co). Inorg. Chim. Acta 2009, 362, 2020–2026. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Ekennia, A.C.; Mogwase, B.M.S.; Olubiyi, O.O.; Hosten, E. Palladium(II) and platinum(II) complexes of N-butyl-N-phenyldithiocarbamate: Synthesis, characterization, biological activities and molecular docking studies. Inorg. Chim. Acta 2016, 450, 69–80. [Google Scholar] [CrossRef]
- Konarev, D.V.; Kovalevsky, A.Y.; Otsuka, A.; Saito, G.; Lyubovskaya, R.N. Neutral and ionic complexes of C60 with metal dibenzyldithiocarbamates. Reversible dimerization of C60•− in ionic multicomponent complex [CrI(C6H6)2•+]·(C60•−)·0.5[Pd(dbdtc)2]. Inorg. Chem. 2005, 44, 9547–9553. [Google Scholar] [CrossRef]
- Gupta, A.N.; Kumar, V.; Singh, V.; Manar, K.K.; Drew, M.G.B.; Singh, N. Intermolecular anagostic interactions in group 10 metal dithiocarbamates. CrystEngComm 2014, 16, 9299–9307. [Google Scholar] [CrossRef]
- Yadav, M.K.; Rajput, G.; Prasad, L.B.; Drew, M.G.B.; Singh, N. Rare intermolecular M···H–C anagostic interactions in homoleptic Ni(II)–Pd(II) dithiocarbamate complexes. New J. Chem. 2015, 39, 5493–5499. [Google Scholar] [CrossRef]
- Genre, C.; Levasseur-Theriault, G.; Reber, C. Emitting-state properties of square-planar dithiocarbamate complexes of palladium(II) and platinum(II) probed by pressure-dependent luminescence spectroscopy. Can. J. Chem. 2009, 87, 1625–1635. [Google Scholar] [CrossRef]
- Shaheen, F.; Badshah, A.; Anjum, S.; Saqib, A. Bis(piperidine-1-dithiocarbamato-κ2S,S′)palladium(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m329–m330. [Google Scholar] [CrossRef]
- Shahzadi, S.; Ali, S.; Badshah, A.; Shaheen, F.; Ahmed, F.; Fettouhi, M. Synthesis and crystal structure of bis(4-methylpiperidine-dithiocarbamato-S,S′)-palladium(II). J. Chem. Cryst. 2006, 36, 567–570. [Google Scholar] [CrossRef]
- Mukherjee, T.; Sarkar, S.; Marek, J.; Zangrando, E.; Chattopadhyay, P. Palladium(II) complexes of dithiocarbamic acids: Synthesis, characterization, crystal structure and DNA binding study. Transit. Met. Chem. 2012, 37, 155–161. [Google Scholar] [CrossRef]
- Torres-Huerta, A.; Höpfl, H.; Tlahuext, H.; Hernández-Ahuactzi, I.F.; Sánchez, M.; Reyes-Martínez, R.; Morales-Morales, D. Dinuclear macrocyclic palladium dithiocarbamates derived from the homologous series of aliphatic 1,x-diamines (x = 4–10). Eur. J. Inorg. Chem. 2013, 2013, 61–69. [Google Scholar] [CrossRef]
- Webber, P.R.A.; Drew, M.G.B.; Hibbert, R.; Beer, P.D. Transition metal-directed self-assembly of calix[4]arene based dithiocarbamate ligands. Dalton Trans. 2004, 1127–1135. [Google Scholar] [CrossRef]
- Sherwood, R.; de Rivera, F.G.; Wan, J.H.; Zhang, Q.; White, A.J.P.; Rossell, O.; Hogarth, G.; Wilton-Ely, J.D.E.T. Multimetallic complexes based on a diphosphine-dithiocarbamate “janus” ligand. Inorg. Chem. 2015, 54, 4222–4230. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Pandey, R.; Singh, R.; Srivastava, N.; Maiti, B.; Saha, S.; Li, P.; Xu, Q.; Pandey, D.S. Heteroleptic dipyrrinato complexes containing 5-ferrocenyldipyrromethene and dithiocarbamates as coligands: Selective chromogenic and redox probes. Inorg. Chem. 2012, 51, 8916–8930. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, V.; Drew, M.G.B.; Singh, N. Syntheses, crystal structures and photoluminescent properties of new heteroleptic Ni(II) and Pd(II) complexes of ferrocene functionalized dithiocarbamate-and dipyrromethene ligands. Inorg. Chem. Commun. 2013, 37, 151–154. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.-F.; Yu, S.-Y. Syntheses, characterization and crystal structures of dithiocarbamate-based mononuclear palladium(II) complexes. Chin. J. Struct. Chem. 2017, 36, 1185–1192. [Google Scholar] [CrossRef]
- Wiench, J.W.; Michon, C.; Ellern, A.; Hazendonk, P.; Iuga, A.; Angelici, R.J.; Pruski, M. Solid-state NMR investigations of the immobilization of a BF4− salt of a palladium(II) complex on silica. J. Am. Chem. Soc. 2009, 131, 11801–11810. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.D.; Wu, X.H. Bimetallic DppfM(II) (M = Pt and Pd) dithiocarbamate complexes: Synthesis, characterization, and anticancer activity. J. Chem. Res. 2019, 43, 437–442. [Google Scholar] [CrossRef]
- Jantan, K.A.; Kwok, C.Y.; Chan, K.W.; Marchiò, L.; White, A.J.P.; Deplano, P.; Serpe, A.; Wilton-Ely, J.D.E.T. From recovered metal waste to high-performance palladium catalysts. Green Chem. 2017, 19, 5846–5853. [Google Scholar] [CrossRef] [Green Version]
- Knight, E.R.; Leung, N.H.; Lin, Y.H.; Cowley, A.R.; Watkin, D.J.; Thompson, A.L.; Hogarth, G.; Wilton-Ely, J.D.E.T. Multimetallic arrays: Symmetrical bi-, tri- and tetrametallic complexes based on the group 10 metals and the functionalisation of gold nanoparticles with nickel-phosphine surface units. Dalton Trans. 2009, 3688–3697. [Google Scholar] [CrossRef]
- Oliver, K.; White, A.J.P.; Hogarth, G.; Wilton-Ely, J.D.E.T. Multimetallic complexes of group 10 and 11 metals based on polydentate dithiocarbamate ligands. Dalton Trans. 2011, 40, 5852–5864. [Google Scholar] [CrossRef]
- Khan, H.; Badshah, A.; Zia-ur-Rehman; Said, M.; Murtaza, G.; Shah, A.; Butler, I.S.; Ahmed, S.; Fontaine, F.-G. New dimeric and supramolecular mixed ligand palladium(II) dithiocarbamates as potent DNA binders. Polyhedron 2012, 39, 1–8. [Google Scholar] [CrossRef]
- Prakasam, B.A.; Lahtinen, M.; Peuronen, A.; Muruganandham, M.; Kolehmainen, E.; Haapaniemi, E.; Sillanpaa, M. Synthesis, NMR spectral and structural studies on mixed ligand complexes of Pd(II) dithiocarbamates: First structural report on palladium(II) dithiocarbamate with SCN− ligand. J. Mol. Struct. 2016, 1108, 195–202. [Google Scholar] [CrossRef]
- Shaheen, F.; Badshah, A.; Gielen, M.; Gieck, C.; de Vos, D. Synthesis, characterization and in vitro cytotoxicity of palladium(II) complexes with mixed ligands. X-ray diffraction study of C31H36ClNPPdS2. Appl. Organomet. Chem. 2007, 21, 633–640. [Google Scholar] [CrossRef]
- Shaheen, F.; Najam-Ul-Haq, M.; Wurst, K.; Badshah, A.; Ali, S. Chloro(4-methylpiperidine-1-dithiocarbamato-κ2S:S′)(triphenylphosphine-κP)palladium(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m136–m137. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, F.; Badshah, A.; Gielen, M.; Croce, G.; Florke, U.; de Vos, D.; Ali, S. In vitro assessment of cytotoxicity, anti-inflammatory, antifungal properties and crystal structures of metallacyclic palladium(II) complexes. J. Organomet. Chem. 2010, 695, 315–322. [Google Scholar] [CrossRef]
- Florke, U. Chloro-(3,4-dihydroisoquinoline-2(1H)-carbodithioato)-(tris(2-methylphenyl)phosphine)-palladium(ii). CSD Commun. 2016. [Google Scholar] [CrossRef]
- Nawaz, H.; Waseem, A.; Nafees, M.; Arshad, M.N.; Rashid, U. Synthesis, characterization, cytotoxicity and computational studies of new phosphine and carbodithioate based palladium(II) complexes. Appl. Organomet. Chem. 2017, 31, e3771. [Google Scholar] [CrossRef]
- Khan, S.Z.; Butler, I.S.; Belanger-Gariepy, F. New ternary palladium(II) complexes: Synthesis, characterization, in vitro anticancer and antioxidant activities. Inorg. Chem. Commun. 2019, 105, 140–146. [Google Scholar] [CrossRef]
- Khan, S.Z.; Amir, M.K.; Ullah, I.; Aamir, A.; Pezzuto, J.M.; Kondratyuk, T.; Bélanger-Gariepy, F.; Ali, A.; Khan, S.; Zia-ur-Rehman. New heteroleptic palladium(II) dithiocarbamates: Synthesis, characterization, packing and anticancer activity against five different cancer cell lines. Appl. Organomet. Chem. 2016, 30, 392–398. [Google Scholar] [CrossRef]
- Khan, S.Z.; Amir, M.K.; Abbasi, R.; Tahir, M.N.; Zia-ur-Rehman. New 3D and 2D supramolecular heteroleptic palladium(II) dithiocarbamates as potent anticancer agents. J. Coord. Chem. 2016, 69, 2999–3009. [Google Scholar] [CrossRef]
- Khan, S.Z.; Amir, M.K.; Naseer, M.M.; Abbasi, R.; Mazhar, K.; Tahir, M.N.; Awan, I.Z.; Zia-ur-Rehman. Heteroleptic Pd(II) dithiocarbamates: Synthesis, characterization, packing and in vitro anticancer activity against HeLa cell line. J. Coord. Chem. 2015, 68, 2539–2551. [Google Scholar] [CrossRef]
- Khan, S.Z.; Amir, M.K.; Ullah, I.; Akhter, M.S.; Bélanger-Gariepy, F. Heteroleptic palladium(II) dithiocarbamates: Synthesis, characterization and in vitro biological screening. J. Mol. Struct. 2018, 1156, 564–570. [Google Scholar] [CrossRef]
- Khan, H.; Badshah, A.; Said, M.; Murtaza, G.; Ahmad, J.; Jean-Claude, B.J.; Todorova, M.; Butler, I.S. Anticancer metallopharmaceutical agents based on mixed-ligand palladium(II) complexes with dithiocarbamates and tertiary organophosphine ligands. Appl. Organomet. Chem. 2013, 27, 387–395. [Google Scholar] [CrossRef]
- Khan, H.; Badshah, A.; Murtaz, G.; Said, M.; Neuhausen, C.; Todorova, M.; Jean-Claude, B.J.; Butler, I.S. Synthesis, characterization and anticancer studies of mixed ligand dithiocarbamate palladium(II) complexes. Eur. J. Med. Chem. 2011, 46, 4071–4077. [Google Scholar] [CrossRef]
- Khan, H.; Badshah, A.; Said, M.; Murtaza, G.; Sirajuddin, M.; Ahmad, J.; Butler, I.S. Synthesis, structural characterization and biological screening of heteroleptic palladium(II) complexes. Inorg. Chim. Acta 2016, 447, 176–182. [Google Scholar] [CrossRef]
- Chan, E.Y.Y.; Zhang, Q.-F.; Sau, Y.-K.; Lo, S.M.F.; Sung, H.H.Y.; Williams, I.D.; Haynes, R.K.; Leung, W.-H. Chiral bisphosphinite metalloligands derived from a P-chiral secondary phosphine oxide. Inorg. Chem. 2004, 43, 4921–4926. [Google Scholar] [CrossRef]
- Serrano, J.L.; García, L.; Pérez, J.; Pérez, E.; Galiana, J.M.; García, J.; Martínez, M.; Sánchez, G.; da Silva, I. New cyclometallated precursors of unsubstituted N-phenylpyrazole [{Pd(phpz)(μ-X)}2] (X = AcO or OH) and study of their reactivity towards selected ligands. Dalton Trans. 2011, 40, 156–168. [Google Scholar] [CrossRef]
- Kondrashov, M.; Fleckhaus, A.; Gritcenko, R.; Wendt, O.F. Crystal structure of (piperidine-1-carbodithioato-κ2S,S)[2-(pyridin-2-yl)phenyl-κ2C1,N]palladium(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2015, 71, m166. [Google Scholar] [CrossRef] [PubMed]
- Lentijo, S.; Miguel, J.A.; Espinet, P. Cyclopalladated complexes of perylene imine: Mononuclear complexes with five- or six-membered metallacycles. Dalton Trans. 2011, 40, 7602–7609. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.-M.; Zhang, Q.-F.; Mak, W.-L.; Sung, H.H.Y.; Williams, I.D.; Leung, W.-H. Bimetallic complexes with porphyrins containing a cyclometalated phenylpyridine group. J. Organomet. Chem. 2005, 690, 253–260. [Google Scholar] [CrossRef]
- Schildbach, D.; Arroyo, M.; Lehmen, K.; Martin-Barrios, S.; Sierra, L.; Villafane, F.; Strohmann, C. [(Piperidinomethyl)silylmethyl] cyclopalladated complexes: Their synthesis, reactivity, and solid state structures. Organometallics 2004, 23, 3228–3238. [Google Scholar] [CrossRef]
- Wang, H.-F.; Yih, K.-H.; Leeb, G.-H.; Huang, S.-L. Synthesis, reactivity and crystal structures of the Palladium(II) complexes with the pyrimidine containing ligand: Crystal structures of [Pd(PPh3)(η1-C4H3N2)(η2-S2CNC4H8)] and [Pd(PPh3)(η1-C4H3N2)(η2-Tp)]. J. Chin. Chem. Soc. 2011, 58, 174–180. [Google Scholar] [CrossRef]
- Kwok, W.-H.; Lu, G.-L.; Rickard, C.E.F.; Roper, W.R.; Wright, L.J. The structurally characterised silyl complexes, Os(κ2-S2CNMe2)-(SiMeCl2)(CO)(PPh3)2 and Os(κ2-S2CNMe2)(SiCl3)(CO)(PPh3)2, which have remarkably unreactive Si–Cl bonds. J. Organomet. Chem. 2006, 691, 2593–2598. [Google Scholar] [CrossRef]
- Albrecht, M.; Kwok, W.-H.; Lu, G.-L.; Rickard, C.E.F.; Roper, W.R.; Salter, D.M.; Wright, L.J. Osmadisiloxane and osmastannasiloxane complexes derived from silanolate complexes of osmium(II). Inorg. Chim. Acta 2005, 358, 1407–1419. [Google Scholar] [CrossRef]
- Möhlen, M.M.; Rickard, C.E.F.; Roper, W.R.; Whittell, G.R.; Wright, L.J. Syntheses, reactions, and structures of osmium(II) stannyl complexes with the simple stannyl ligands −SnH3, −SnH2Me, and −SnHMe2. Inorg. Chim. Acta 2007, 360, 1287–1297. [Google Scholar] [CrossRef]
- Lu, G.L.; Möhlen, M.M.; Rickard, C.E.; Roper, W.R.; Wright, L.J. A cyclic osmastannyl complex, Os(κ2(Sn,P)-SnMe2C6H4PPh2)(κ2-S2CNMe2)(CO)(PPh3) derived from the osmastannol complex, Os(SnMe2OH)(κ2-S2CNMe2)(CO)(PPh3)2. Inorg. Chim. Acta 2005, 358, 4145–4155. [Google Scholar] [CrossRef]
- Möhlen, M.M.; Rickard, C.E.F.; Roper, W.R.; Whittell, G.R.; Wright, L.J. Syntheses, reactions, and structures of osmium(II) distannyl complexes, LnOs–SnMe2SnR3 (R = Me, Ph), from reaction between LnOs–SnClMe2 and either LiSnMe3 or KSnPh3. J. Organomet. Chem. 2006, 691, 4065–4075. [Google Scholar] [CrossRef]
- Karim, M.M.; Abser, M.N.; Hassan, M.R.; Ghosh, N.; Alt, H.G.; Richards, I.; Hogarth, G. Oxidative-addition of thiuram disulfides to osmium(0): Synthesis of cis-[Os(CO)2(S2CNR2)2] (R = Me, Et, Cy, CH2CH2OMe) and molecular structures of cis-[Os(CO)2(S2CNMe2)2] and [(MeOCH2CH2)2NCS]2. Polyhedron 2012, 42, 84–88. [Google Scholar] [CrossRef]
- Bhoumik, N.C.; Saha, T.K.; Ghosh, S.; Nesterov, V.N.; Richmond, M.G.; Kabir, S.E. Facile Os-Os bond cleavage in the reactions of [Os3(CO)10(NCMe)2] and [Os3(CO)10(μ-H)2] with tetramethylthiuram disulfide (tmtd): Syntheses and crystal structures of new polynuclear osmium carbonyl complexes containing a dimethyldithiocarbamate ligand(s). J. Organomet. Chem. 2020, 911, 121133. [Google Scholar] [CrossRef]
- Garcia-Orozco, I.; Pastor, C.J.; Souto, B.; Delgado, E.; Hernandez, E.; Alvarez-Toledano, C. Nonacarbonyl-μ3-N,N-diethyldithiocarbamato-κ3S:S:S′-μ-hydrido-triosmium(3 Os-Os). Acta Crystallogr. Sect. E Crystallogr. Commun. 2005, 61, m1644–m1645. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-Q. (N,N′-Diethyldithiocarbamato-κ2S,S′)bis[2-(2-pyridyl)phenyl-κ 2C1,N]iridium(III). Acta Crystallogr. Sect. E Crystallogr. Commun. 2007, 63, m2078. [Google Scholar] [CrossRef]
- Lau, M.-K.; Cheung, K.-M.; Zhang, Q.-F.; Song, Y.; Wong, W.-T.; Williams, I.D.; Leung, W.-H. Iridium(III) and rhodium(III) cyclometalated complexes containing sulfur and selenium donor ligands. J. Organomet. Chem. 2004, 689, 2401–2410. [Google Scholar] [CrossRef]
- Wang, X.-M.; Qiang, J.-Y.; Jia, A.-Q.; Tong, B.; Zhang, Q.-F. Syntheses, crystal structures and phosphorescence properties of cyclometalated iridium(III) bis(pyridylbenzaldehyde) complexes with dithiolate ligands. Z. Naturforsch. B Chem. Sci. 2017, 72, 941–946. [Google Scholar] [CrossRef]
- Lu, G.-Z.; Tu, Z.-L.; Liu, L.; Zhang, W.-W.; Zheng, Y.-X. Fast synthesis of iridium (iii) complexes with sulfur-containing ancillary ligand for high-performance green OLEDs with EQE exceeding 31%. J. Mater. Chem. C 2019, 7, 7273–7278. [Google Scholar] [CrossRef]
- Lu, G.-Z.; Wu, R.; Liu, L.; Zhou, L.; Zheng, Y.-X.; Zhang, W.-W.; Zuo, J.-L.; Zhang, H. A series of red iridium(III) complexes using flexible dithiocarbamate derivatives as ancillary ligands for highly efficient phosphorescent OLEDs. Mater. Chem. Front. 2019, 3, 860–866. [Google Scholar] [CrossRef]
- Lu, G.Z.; Su, N.; Yang, H.Q.; Zhu, Q.; Zhang, W.-W.; Zheng, Y.-X.; Zhou, L.; Zuo, J.-L.; Chen, Z.-X.; Zhang, H.-J. Rapid room temperature synthesis of red iridium(iii) complexes containing a four-membered Ir–S–C–S chelating ring for highly efficient OLEDs with EQE over 30%. Chem. Sci. 2019, 10, 3535–3542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, N.; Li, F.-L.; Zheng, Y.-X. Four-membered red iridium(III) complexes with Ir–S–C–S structures for efficient organic light-emitting diodes. J. Mater. Chem. C 2020, 8, 7411–7416. [Google Scholar] [CrossRef]
- Lu, G.-Z.; Li, X.; Liu, L.; Zhou, L.; Zheng, Y.-X.; Zhang, W.-W.; Zuo, J.-L.; Zhang, H. Efficient phosphorescent red iridium(III) complexes containing a four-membered Ir–S–C–S ring backbone and large hindered spacers for high-performance OLEDs. J. Mater. Chem. C 2019, 7, 3862–3868. [Google Scholar] [CrossRef]
- Mei, Q.; Chen, C.; Tian, R.; Yang, M.; Tong, B.; Hua, Q.; Shi, Y.; Fan, Q.; Ye, S. Highly efficient orange phosphorescent organic light-emitting diodes based on an iridium(III) complex with diethyldithiocarbamate (S^S) as the ancillary ligand. RSC Adv. 2016, 6, 64003–64008. [Google Scholar] [CrossRef]
- Tong, B.; Ma, P.; Zhang, M.; Liu, Y.; Mei, Q.; Zhang, Q.-F. Phosphorescent iridium(III) carbodithioate complex for the detection of Hg2+ and acetonitrile. Inorg. Chem. Commun. 2013, 37, 121–126. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Zolotarev, A.A.; Ivanov, A.Y.; Smirnov, S.N.; Baichurin, R.I.; Balashev, K.P. Complexes of Ir(III) and Pt(II) with cyclometallated 2-phenylbenzothiazole and chelating diethyldithiocarbamate and o-ethyldithiocarbonate ions: Structures and optical and electrochemical properties. Russ. J. Coord. 2016, 42, 178–186. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Zolotarev, A.A.; Balashev, K.P. Influence of ligand donor-acceptor properties on the structure, optical, and electrochemical characteristics of Ir(III) complexes with cyclometallated 2-phenylbenzotiazole. Russ. J. Gen. Chem. 2016, 86, 2508–2514. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Z.; Yang, J.; Zhang, S. The synthesis and crystal structure of bis(2-(benzo[d]thiazol-2-yl)-5-methylbenzen-1-ido-κ2C,N)-(N,N′-diethyldithiocarbamato-κ2S,S′)iridium(III), C33H30N3S4Ir. Z. Kristallogr. New Cryst. Struct. 2019, 234, 1173–1176. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Qin, J.; Gao, J.; You, H.; Ma, D. Synthesis, structure, electrochemistry, photophysics and electroluminescence of 1,3,4-oxadiazole-based ortho-metalated iridium(III) complexes. J. Organomet. Chem. 2006, 691, 3519–3530. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Li, M.; Qin, J.; Gao, J.; You, H.; Ma, D. Supramolecular architectures, photophysics, and electroluminescence of 1,3,4-oxadiazole-based iridium(III) complexes: From μ-dichloro bridged dimer to mononuclear complexes. Cryst. Growth Des. 2007, 7, 39–46. [Google Scholar] [CrossRef]
- Clark, G.R.; Ferguson, L.A.; McIntosh, A.E.; Söhnel, T.; Wright, L.J. Functionalization of metallabenzenes through nucleophilic aromatic substitution of hydrogen. J. Am. Chem. Soc. 2010, 132, 13443–13452. [Google Scholar] [CrossRef]
- Scharwitz, M.; van Almsick, T.; Sheldrick, W.S. Carbonyl(diethyldithioarbamato)(η5-pentamethylcyclopentadienyl)iridium(III) trifluoromethanesulfonate. Acta Crystallogr. Sect. E Crystallogr. Commun. 2007, E63, m105–m107. [Google Scholar] [CrossRef]
- Scharwitz, M.; Oppel, I.M.; Sheldrick, W.S. Bis(μ-diethyldithiocarbamato)bis[(η5-pentamethylcyclopentadienyl)iridium(III)] bis(perchlorate). Acta Crystallogr. Sect. E Crystallogr. Commun. 2007, 63, m2065–m2066. [Google Scholar] [CrossRef]
- Kotera, M.; Sekioka, Y.; Suzuki, T. Structural versatility of 5-methyltetrazolato complexes of (η5-pentamethylcyclopentadienyl)iridium(III) incorporating 2,2′-bipyridine, N,N-dimethyldithiocarbamate, or 2-pyridinethiolate ligands. Inorg. Chem. 2008, 47, 3498–3508. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Mouatassim, B.E.; Mortini, F.; Belanger-Gariepy, F.; Lough, A. Reactions of (η5-C5Me5)Ir(PMe3)(SH)2 and (η5-C5Me5)Ir(PMe3)(SH)(H) with thionylaniline (PhNSO) to give novel iridium S3O and S2O complexes. Organometallics 2007, 26, 4229–4233. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Suzuki, T.; Mayer, J.M.; Kojima, M. Reactivities of the N-atom-inserted ligands, NSC(NR2)S2− and SN=C(NR2)S2−, in iridium(III) complexes. Chem. Lett. 2011, 40, 831–833. [Google Scholar] [CrossRef]
- Onwudiwe, D.C.; Mugo, J.N.; Hrubaru, M.; Hosten, E. Bis diallyl dithiocarbamate Pt(II) complex: Synthesis, characterization, thermal decomposition studies, and experimental and theoretical studies on its crystal structure. J. Sulfur Chem. 2015, 36, 36–47. [Google Scholar] [CrossRef]
- Zaeva, A.S.; Ivanov, M.A.; Gerasimenko, A.V.; Ivanov, A.V.; Antzutkin, O.N. Dialkyldithiocarbamate platinum(II) complexes of [Pt(S2CNR2)2] (R = iso-C3H7, iso-C4H9): Preparation, 13C CP-MAS NMR, molecular structure, supramolecular self-assembly and thermal behaviour. Polyhedron 2020, 175, 114166. [Google Scholar] [CrossRef]
- Meletov, K.P.; Konarev, D.V. Raman study of the pressure-induced phase transitions in the molecular donor–acceptor complex {Pt(dbdtc)2}C60. Chem. Phys. Lett. 2012, 553, 21–25. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Younas, M.; Rehman, A.; Altaf, M.; Khan, M.Y.; Al-Ahmed, A.; Ahmad, S.; Isab, A.A. Synthesis and utilization of platinum(II) dialkyldithiocarbamate precursors in aerosol assisted chemical vapor deposition of platinum thin films as counter electrodes for dye-sensitized solar cells. Polyhedron 2019, 166, 186–195. [Google Scholar] [CrossRef]
- Poirier, S.; Rahmani, F.; Reber, C. Large d–d luminescence energy variations in square-planar bis(dithiocarbamate) platinum(II) and palladium(II) complexes with near-identical MS4 motifs: A variable-pressure study. Dalton Trans. 2017, 46, 5279–5287. [Google Scholar] [CrossRef]
- Poirier, S.; Roberts, R.J.; Le, D.; Leznoff, D.B.; Reber, C. Interpreting effects of structure variations induced by temperature and pressure on luminescence spectra of platinum(II) bis(dithiocarbamate) compounds. Inorg. Chem. 2015, 54, 3728–3735. [Google Scholar] [CrossRef] [PubMed]
- Leka, Z.; Vojta, D.; Kosovic, M.; Latinovic, N.; Dakovic, M.; Visnjevac, A. Syntheses, structures and antifungal activities of novel Co, Mo and Pt complexes with triammonium N,N-diacetatedithiocarbamate. Polyhedron 2014, 80, 233–242. [Google Scholar] [CrossRef]
- Liebing, P.; Witzorke, J.; Oehler, F.; Schmeide, M. Dithiocarbamatocarboxylate (DTCC) ligands-building blocks for hard/soft-heterobimetallic coordination polymers. Inorg. Chem. 2020, 59, 2825–2832. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Fatokun, A.A.; Andrew, F.P. Synthesis, characterization and anti-cancer studies of Mn(II), Cu(II), Zn(II) and Pt(II) dithiocarbamate complexes-crystal structures of the Cu(II) and Pt (II) complexes. Inorg. Chim. Acta 2020, 504, 119431. [Google Scholar] [CrossRef]
- Andrew, F.P.; Ajibade, P.A. Synthesis, characterization and anticancer studies of bis(1-phenylpiperazine dithiocarbamato) Cu(II), Zn(II) and Pt(II) complexes: Crystal structures of 1-phenylpiperazine dithiocarbamato-S,S′ zinc(II) and Pt(II). J. Mol. Struct. 2018, 1170, 24–29. [Google Scholar] [CrossRef]
- Ivanov, M.A.; Zaeva, A.S.; Gerasimenko, A.V.; Ivanov, A.V. Platinum(II) cyclo-hexamethylenedithiocarbamate complex, [Pt{S2CN(CH2)6}2] and its solvated form, [Pt{S2CN(CH2)6}2]·CHCl3: Crystal and molecular structures, 13C CP/MAS NMR data, and thermal behavior. Russ. J. Coord. Chem. 2013, 39, 764–770. [Google Scholar] [CrossRef]
- Keter, F.K.; Guzei, I.A.; Darkwa, J. N-heterocyclic dithiocarbamate platinum(II) complexes: Unexpected transformation of dithiocarbamate to oxodithiocarbonate in phosphinoplatinum complexes in solution. Inorg. Chem. Commun. 2013, 27, 60–63. [Google Scholar] [CrossRef]
- Montagner, D.; Miguel, P.J.S. Unique Pt5 metallacycle: [PtIICl(pyrrolidinedithiocarbamate)]5. Dalton Trans. 2011, 40, 10809–10811. [Google Scholar] [CrossRef] [Green Version]
- Isab, A.A.; Ali, M.A.J.; Sharif, S.; Khan, I.U.; Kang, S.K.; Khalid, T.; Saleem, M.; Ahmad, S. Synthesis, crystal structure and antimicrobial studies of chlorido(dimethylsulfoxide-κS)(pyrrolidinedithiocarbamato-κ2S,S′) platinum(II). Inorg. Chem. Commun. 2011, 14, 1962–1965. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Reger, D.L. Chloro-(diethylcarbamodithioato)-(triethylphosphine)-platinum. CSD Commun. 2019. [Google Scholar] [CrossRef]
- Amir, M.K.; Hayat, F.; Khan, S.Z.; Hogarth, G.; Kondratyuk, T.; Pezzuto, J.M.; Tahir, M.N. Monofunctional platinum(II) dithiocarbamate complexes: Synthesis, characterization and anticancer activity. RSC Adv. 2016, 6, 110517–110524. [Google Scholar] [CrossRef]
- Imran, M.; Kondratyuk, T.; Belanger-Gariepy, F. New ternary platinum(II) dithiocarbamates: Synthesis, characterization, anticancer, DNA binding and DNA denaturing studies. Inorg. Chem. Commun. 2019, 103, 12–20. [Google Scholar] [CrossRef]
- Verron, R.; Achard, T.; Seguin, C.; Fournel, S.; Bellemin-Laponnaz, S. Synthesis and characterization of n-heterocyclic carbene dithiocarbamate platinum complexes with antitumoral activity. Eur. J. Inorg. Chem. 2020, 2020, 2552–2557. [Google Scholar] [CrossRef]
- Forniés, J.; Sicilia, V.; Casas, J.M.; Martín, A.; López, J.A.; Larraz, C.; Borja, P.; Ovejero, C. Pt–Ag clusters and their neutral mononuclear Pt(II) starting complexes: Structural and luminescence studies. Dalton Trans. 2011, 40, 2898–2912. [Google Scholar] [CrossRef]
- Expósito, J.E.; Álvarez-Paíno, M.; Aullón, G.; Miguel, J.A.; Espinet, P. Higher fluorescence in platinum(IV) orthometallated complexes of perylene imine compared with their platinum(II) or palladium(II) analogues. Dalton Trans. 2015, 44, 16164–16176. [Google Scholar] [CrossRef] [Green Version]
- Paziresh, S.; Aghakhanpour, R.B.; Fuertes, S.; Sicilia, V.; Hosseini, F.N.; Nabavizadeh, S.M. A double rollover cycloplatinated(II) skeleton: A versatile platform for tuning emission by chelating and non-chelating ancillary ligand systems. Dalton Trans. 2019, 48, 5713–5724. [Google Scholar] [CrossRef]
- Rheingold, A.L.; Garza, D. (Diethylcarbamodithioato)-(isobutyl)-(triethylphosphine)-platinum(II). CSD Commun. 2015. [Google Scholar] [CrossRef]
- Burt, L.K.; Hill, A.F. Heterobimetallic μ2-carbido complexes of platinum and tungsten. Dalton Trans. 2020, 49, 8143–8161. [Google Scholar] [CrossRef]
- Fortuño, C.; Martín, A.; Mastrorilli, P.; Latronico, M.; Petrelli, V.; Todisco, S. Stable mixed-valence diphenylphosphanido bridged platinum(II)–platinum(IV) complexes. Dalton Trans. 2020, 49, 4935–4955. [Google Scholar] [CrossRef]
- Heard, P.J.; Kite, K.; Nielsen, J.S.; Tocher, D.A. Trimethylplatinum(IV) complexes of dithiocarbamato ligands: An experimental NMR study on the barrier to C–N bond rotation. J. Chem. Soc. Dalton Trans. 2000, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Evans, D.F. The determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance. J. Chem. Soc. 1959, 2003–2005. [Google Scholar] [CrossRef]
- Brustolin, L.; Nardon, C.; Pettenuzzo, N.; Zuin Fantoni, N.; Quarta, S.; Chiara, F.; Gambalunga, A.; Trevisan, A.; Marchiò, L.; Pontisso, P.; et al. Synthesis, chemical characterization and cancer cell growth-inhibitory activities of Cu(II) and Ru(III) aliphatic and aromatic dithiocarbamato complexes. Dalton Trans. 2018, 47, 15477–15486. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.R.; Stephenson, T.A. Metal complexes of sulphur ligands. Part 15. Reaction of bis[(η-arene)dichlororuthenium] and bis[dichloro(η-pentamethylcyclopentadienyl)metal] complexes of rhodium and iridium with various dithioacid ligands. J. Chem. Soc. Dalton Trans. 1978, 486–495. [Google Scholar] [CrossRef]
- Forth, C.S. Studies on Sulfur-Capped Osmium and Ruthenium Clusters with Focus on Dynamics; University of London: London, UK, 2003. [Google Scholar]
- Van Gaal, H.L.M.; Diesveld, J.W.; Pijpers, F.W.; Van der Linden, J.G.M. Carbon-13 NMR spectra of dithiocarbamates. Chemical shifts, carbon-nitrogen stretching vibration frequencies and. pi.-bonding in the NCS2 fragment. Inorg. Chem. 1979, 18, 3251–3260. [Google Scholar] [CrossRef]
- Ursini, C.V. Platinum-195 nuclear magnetic resonance of organometallic compounds. Quím. Nova 1997, 20, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Trzaska, S. Cisplatin. Chem. Eng. News 2005, 83, 52. [Google Scholar] [CrossRef]
- Kamoon, R.A.; Nadhum, S.A.; Mohammed, M.H. Dithiocarbamates derivatives as anticancer agents: A Review. Ann. Trop. Med. Public Health 2020, 23, S19. [Google Scholar] [CrossRef]
- Moharana, P.; Ghosh, D.; Paira, R. Drive to organoruthenium and organoiridium complexes from organoplatinum: Next-generation anticancer metallotherapeutics. Inorg. Chem. Commun. 2021, 124, 108364. [Google Scholar] [CrossRef]
- Hussain, S.; Bukhari, I.H.; Ali, S.; Shahzadi, S.; Shahid, M.; Munawar, K.S. Synthesis and spectroscopic and thermogravimetric characterization of heterobimetallic complexes with Sn(IV) and Pd(II); DNA binding, alkaline phosphatase inhibition and biological activity studies. J. Coord. Chem. 2015, 68, 662–677. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, S.; Shahzadi, S.; Shahid, M.; Tahir, A.A.; Abbas, S.M.; Riaz, M.; Ahmad, I.; Hussain, I. Multinuclear (Sn/Pd) complexes with disodium 2,2′-(dithiocarboxyazanediyl)diacetate hydrate; Synthesis, characterization and biological activities. J. Coord. Chem. 2017, 70, 4070–4092. [Google Scholar] [CrossRef]
- Odularu, A.T.; Ajibade, P.A.; Mbese, J.Z.; Oyedeji, O.O. Developments in platinum-group metals as dual antibacterial and anticancer agents. J. Chem. 2019, 2019, 5459461. [Google Scholar] [CrossRef]
- Anant, R.; Kapdi, A.R.; Fairlamb, I.J.S. Anti-cancer palladium complexes: A focus on PdX2L2, palladacycles and related complexes. Chem. Soc. Rev. 2014, 43, 4751–4777. [Google Scholar] [CrossRef]
- Fanelli, M.; Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M.; Paoli, P. New trends in platinum and palladium complexes as antineoplastic agents. Coord. Chem. Rev. 2016, 310, 41–79. [Google Scholar] [CrossRef]
- Alam, M.N.; Huq, F. Comprehensive review on tumour active palladium compounds and structure–activity relationships. Coord. Chem. Rev. 2016, 316, 36–67. [Google Scholar] [CrossRef]
- Amir, M.K.; Zebkhan, S.; Hayat, F.; Hassan, A.; Butler, I.S.; Zia-ur-Rehman. Anticancer activity, DNA-binding and DNA-denaturing aptitude of palladium(II) dithiocarbamates. Inorg. Chim. Acta 2016, 451, 31–40. [Google Scholar] [CrossRef]
- Kang, M.S.; Choi, E.K.; Choi, D.H.; Ryu, S.Y.; Lee, H.H.; Kang, H.C.; Koh, J.T.; Kim, O.S.; Hwang, Y.C.; Yoon, S.J.; et al. Antibacterial activity of pyrrolidine dithiocarbamate. FEMS Microbiol. Lett. 2008, 280, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.K.; Lee, H.H.; Kang, M.S.; Kim, B.G.; Lim, H.S.; Kim, S.M.; Kang, I.C. Potentiation of bacterial killing activity of zinc chloride by pyrrolidine dithiocarbamate. J. Microbiol. 2010, 48, 40–43. [Google Scholar] [CrossRef]
- Yeo, C.I.; Tiekink, E.R.T.; Chew, J. Insights into the Antimicrobial Potential of Dithiocarbamate Anions and Metal-Based Species. Inorganics 2021, 9, 48. [Google Scholar] [CrossRef]
- Giovagnini, L.; Marzano, C.; Bettio, F.; Fregona, D. Mixed complexes of Pt(II) and Pd(II) with ethylsarcosinedithiocarbamate and 2-/3-picoline as antitumor agents. J. Inorg. Biochem. 2005, 99, 2139–2150. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Afzaal, M.; O’Brien, P. Precursor Chemistry for Main Group Elements in Semiconducting Materials. Chem. Rev. 2010, 110, 4417–4446. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.E.; Carmalt, C.J. Solution based CVD of main group materials. Chem. Soc. Rev. 2016, 45, 1036–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredrick, G.; Erling, R. On the sulfides, selenides, and tellurides of palladium. Acta Chem. Scand. 1956, 10, 1620–1634. [Google Scholar] [CrossRef] [Green Version]


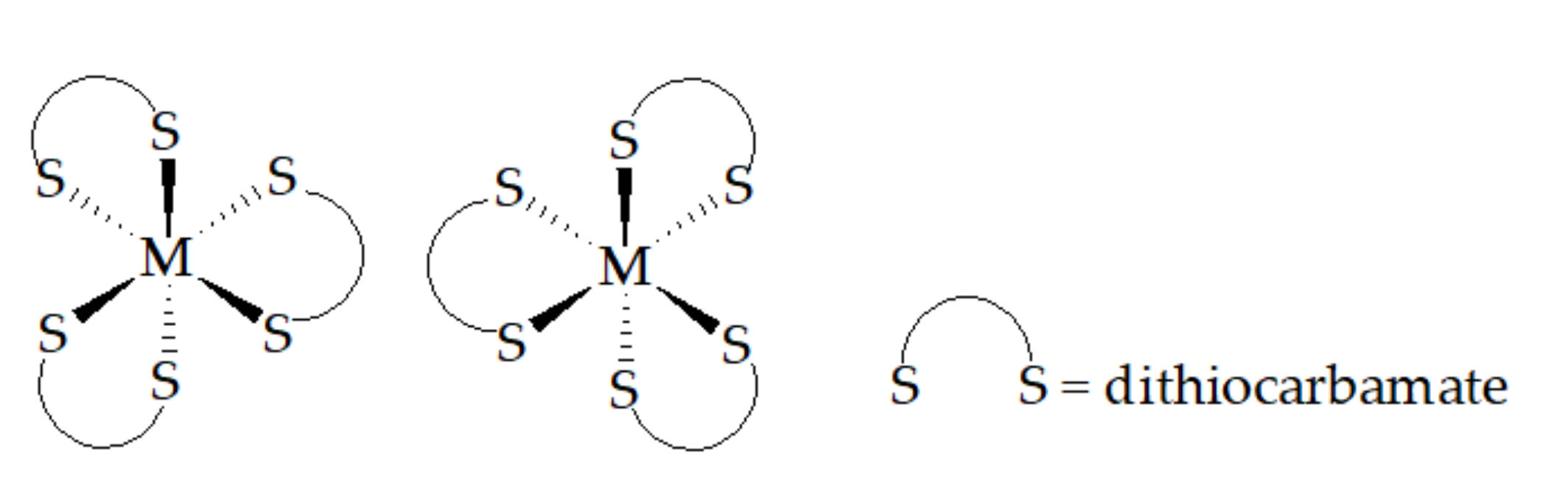

| −S2CNRR′ | ||
|---|---|---|
| Ligand | R | R′ |
| L1 | H | H |
| L2 | H | Me |
| L3 | H | Et |
| L4 | H | Ph |
| L5 | H | p-tolyl |
| L6 | H | C6H4Et-p |
| L7 | H | C6H4Cl-p |
| L8 | Me | Me |
| L9 | Me | CH2C≡CH |
| L10 | Me | CH2CH=CH2 |
| L11 | Me | n-Bu |
| L12 | Me | n-Hex |
| L13 | Me | CH2Ph |
| L14 | Me | Ph |
| L15 | Me | Cy |
| L16 | Me | CH2CH(OMe)2 |
| L17 | Me | CH2COO− |
| L18 | Me | CH2COOH |
| L19 | Me | CH2COOMe |
| L20 | Me | CH2COOEt |
| L21 | Me | CH2COO-t-Bu |
| L22 | Me | CH2CH2OC(=O)CH=CHCOOEt |
| L23 | Me | 2-methyl-1,3-dioxolane |
| L24 | Et | Et |
| L25 | Et | CH2CH2OH |
| L26 | CH2CH2OMe | CH2CH2OMe |
| L27 | CH2CH=CH2 | CH2CH=CH2 |
| L28 | n-Pr | n-Pr |
| L29 | i-Pr | i-Pr |
| L30 | (CH2)3NMe2 | (CH2)3NMe2 |
| L31 | n-Bu | n-Bu |
| L32 | n-Bu | CH2Ph |
| L33 | n-Bu | Ph |
| L34 | CH2(i-Pr) | CH2(i-Pr) |
| L35 | n-Hex | n-Hex |
| L36 | n-Dec | n-Dec |
| L37 | CH2CH(Me)(Et) | CH2CH(Me)(Et) |
| L38 | CH2CH(Et)(n-Bu) | CH2CH(Et)(n-Bu) |
| L39 | CH2Ph | CH2Ph |
| L40 | CH2Ph | CH2C6H4OMe-3 |
| L41 | CH2Ph | CH2C5H4N-3 |
| L42 | CH2Ph | CH2C4H3NMe |
| L43 | CH2Ph | CH2C5H4FeCp |
| L44 | (CH2)2PPh2 | (CH2)2PPh2 |
| L45 | C5H4N-2 | C5H4N-2 |
| L46 | Ph | Ph |
| L47 | Cy | Cy |
| L48 | p-tolyl | p-tolyl |
| L49 | C6H4CF3-p | C6H4CF3-p |
| L50 | C6H4(t-Bu)-p | C6H4(t-Bu)-p |
| L51 | CH2COO− | CH2COO− |
| L52 | CH2COOH | CH2COOH |
| L53 | CH2COOH | CH2COO− |
| L54 | CH2C4H3O | CH2C4H3O |
| L55 | CH2C6H4OH-o | p-tolyl |
| L56 | CH2C(=O)N(H)(CH2)3CH3 | CH2C(=O)N(H)(CH2)3CH3 |
| Crystal | Formulation | REFCODE | Donor Set | Ref. |
|---|---|---|---|---|
| 1 | Ru(L8)3 | OCOVUZ | S6 | [25] |
| 2 | Ru(L20)3 | OCOWEK | S6 | [25] |
| 3 | [Ru(L57)3], CHCl3 | OCOWAG | S6 | [25] |
| 4 | [Ru2(L8)5]BF4, CHCl3, CH3CN | REDCEK | S6 | [32] |
| 5 | [Ru2(L8)5]{[Mo6O19]0.5}, CH3COCH3 | YUJFOZ | S6 | [33] |
| 6 | Ru(L8)2((CH3)2SO)2 | REDBUZ | cis-O2S4 | [32] |
| 7 | Ru(L57)2((CH3)2SO)2 | REDCAG | cis-O2S4 | [32] |
| 8 | (dppb)Ru(L24)2 | EBESOW | cis-P2S4 | [34] |
| 9 | [(dppb)Ru(L63)2], CH2Cl2 | PAZWOD | cis-P2S4 | [35] |
| 10 | (dppb)Ru(L79)2 | EBESIQ | cis-P2S4 | [34] |
| 11 | [Ru(bpy)2(L8)]PF6, 0.75((CH3CH2)2O) | DANDOM | N4S2 | [36] |
| 12 | [Ru(bpy)2(L56)]PF6 | WOHLAH | N4S2 | [37] |
| 13 | [Ru(cyclam)(L24)]BPh4, 0.16(CH2Cl2) | BUPMEF | N4S2 | [38] |
| 14 | [(dppm)2Ru(L9)]PF6, 0.5(CH2Cl2) | COZMIP | P4S2 | [39] |
| 15 | [(dppm)2Ru(L26)]PF6 | HUYPAT | P4S2 | [40] |
| 16 | {[(dppm)2Ru]2(L74)}[BF4]2, 3(CHCl3) | QAVHAX | P4S2 | [41] |
| 17 | [(dppm)2Ru(L74)Pd(PPh3)2][BF4]2, 3(CH2Cl2), (CH3CH2)2O | QONVAR | P4S2 | [42] |
| 18 | (PPh3)2Ru(L8)Cl2, 2(CH2Cl2) | HUPMAH | Cl2P2S2 | [43] |
| 19 | (PPh3)2Ru(L24)Cl2, CH2Cl2 | GUVPIX | Cl2P2S2 | [44] |
| 20 | (PPh3)2Ru(L29)Cl2, CH2Cl2 | GUVPOD | Cl2P2S2 | [44] |
| 21 | [(PPh3)Ru(tptz)(L24)]Cl, 2.5(H2O) | ABIBET | N3PS2 | [45] |
| 22 | (PEt3)Ru(L8)2(CO) | PEBZUS | CPS4 | [46] |
| 23 | (PPh3)Ru{Ge(p-tolyl)2}(L8)2(CO), CH2Cl2 | VEKKIH | CGePS3 | [47] |
| 24 | (PPh3)Ru(κ2(Si,N)-SiPh2NHC5H4N)(L8)(CO) | RADNIU | CNPS2Si | [48] |
| 25 | (PPh3)Ru(κ2(Si,N)-SiPh2OC5H4N)(L8)(CO), 2.5(C6H6) | RADNEQ | CNPS2Si | [48] |
| 26 | (PPh3)Ru(κ2(Si,O)-SiPh2OCCH3O)(L8)(CO), CH2Cl2 | RADNOA | CNPS2Si | [48] |
| 27 | Ru(B(NCH2PPh2)2C6H4)(L24)(CO) | UNAVEM | BCP2S2 | [49] |
| 28 | (PPh3)2Ru(H)(L7)(CO), 0.5(CH3(CH2)4CH3) | OWOKER | CHP2S2 | [50] |
| 29 | (PPh3)2Ru(H)(L8)(CO) | RADMUF | CHP2S2 | [48] |
| 30 | (PPh3)2Ru(H)(L84)(CO) | YOSJEX | CHP2S2 | [51] |
| 31 | (PPh3)2Ru(Si(Cl)Ph2)(L8)(CO) | RADNAM | CP2S2Si | [48] |
| 32 | (PPh3)2Ru(Ge(p-tolyl)3)(L24)(CO), 1.19(CH2Cl2) | VEKKAZ | CGeP2S2 | [47] |
| 33 | (PPh3)2Ru(C(H)=C(H)BMIDA)(L24)(CO), CH3COCH3 | HUDPON | C2P2S2 | [52] |
| 34 | (PPh3)2Ru(C(H)=C(H)C6H4Me-4)(L10)(CO), CH2Cl2 | COZMEL | C2P2S2 | [39] |
| 35 | (PPh3)2Ru(C(H)=C(H)C6H4Me-4)(L26)(CO), 0.3(CH2Cl2) | HUYPEX | C2P2S2 | [40] |
| 36 | (PPh3)2Ru(C(H)=C(H)C6H4Me-4)(L27)(CO), CH2Cl2 | OJEYIM | C2P2S2 | [53] |
| 37 | (PPh3)2Ru(C(H)=C(H)pyrenyl-1)(L84)(CO), 1.5(CH2Cl2) | YOSJIB | C2P2S2 | [51] |
| 38 | (PPh3)2Ru(C(C≡C(Ph)=C(H)Ph)(L84)(CO) | YOSJOH | C2P2S2 | [51] |
| 39 | (PPh3)2Ru(C(C≡CSiMe3)=C(H)SiMe3)(L8)(CO), unknown solvate | KOQPEO | C2P2S2 | [54] |
| 40 | [(PPh3)2Ru(C(C≡CPh)=C(H)Ph)(L65)(CO)]Cl, 1.25(CH2CH2OH) | DOZHOQ | C2P2S2 | [55] |
| 41 | (PPh3)2Ru(C≡CBMIDA)(L8)(CO) | HUDPAZ | C2P2S2 | [52] |
| 42 | (PPh3)2Ru(C≡CBMIDA)(L24)(CO), 0.5(CH2Cl2), unknown solvate | HUDPED | C2P2S2 | [52] |
| 43 | (PPh3)2Ru(X)(L24)(CO) | BOHTOK | C2P2S2 | [56] |
| 44 | (PPh3)2Ru(L24)(CO)Cl, 2.5(C6H6) | HUDQAA | CClP2S2 | [52] |
| 45 | (PPh3)2Ru(L24)(CO)Cl, CH2Cl2 | HUDQEE | CClP2S2 | [52] |
| 46 | (PPh3)2Ru(L24)(CO)Cl, 2(CHCl3), unknown solvate | HUDQII | CClP2S2 | [52] |
| 47 | (PPh3)2Ru(L24)(CO)Cl, CH3OH | HUDQOO | CClP2S2 | [52] |
| 48 | (PPh3)Ru(Ge(p-tolyl)3)(L24)(CO)2 | VEKKED | C2GePS2 | [47] |
| 49 | Ru(L22)(CO)3Cl | ROQQUM | fac-C3ClS2 | [57] |
| 50 | CpRu(PPh3)(L11) | YEDMEA | C3PS2 | [58] |
| 51 | CpRu(PPh3)(L24) | KEZVOB | C3PS2 | [59] |
| 52 | CpRu(PPh3)(L28) | YEDMAW | C3PS2 | [58] |
| 53 | CpRu(PPh3)(L57) | BUYQIW | C3PS2 | [60] |
| 54 | {[CpRu(L24)]2(dppf)}, CH2Cl2 | IZIBAU | C3PS2 | [61] |
| 55 | CpRu(L8)(CO) | QIWNES | C4S2 | [62] |
| 56 | [Cp*Ru(L8)2][N(S=PPh2)2], 0.5(H2O) | PARVUA | C3S4 | [63] |
| 57 | [Cp*Ru(L8)(L24)]Cl, 2CHCl3 | TEYTEX | C3S4 | [64] |
| 58 | Cp*Ru(L8)Cl2 | WEYTUQ | C3Cl2S2 | [65] |
| 59 | Cp*Ru(L24)Cl2 | TEYSUM | C3Cl2S2 | [64] |
| 60 | Cp*Ru(S2C=O)(L8) | TEYTIB | C3S4 | [64] |
| 61 | Cp*Ru(S2C=O)(L24) | TEYTOH | C3S4 | [64] |
| 62 | [Cp*Ru(PPh3)(L8)Cl]Cl, 0.5(H2O), 1.5(CH3CN) | TEYTUN | C3ClPS2 | [64] |
| 63 | [Cp*Ru(PMe3)(L8)Cl]BPh4 | TEYVAV | C3ClPS2 | [64] |
| 64 | [Cp*Ru(L8)Cl]2[SbCl6]2 | WEYVEC | C3Cl2S2 | [65] |
| 65 | [Cp*Ru(L8)(CH3CN)2][PF6]2, 2CH3CN | WEYVAY | C3N2S2 | [65] |
| 66 | Cp*Ru(L8)(CO) | TEYVID | C4S2 | [64] |
| 67 | [(PPh3)2Ru(C(NMe2)OC(NMe2)=S)(L8)]ClO4 | HUPMEL | CP2S3 | [43] |
| 68 | [(PPh3)2Ru(C(NMe2)SC(NMe2)=S)(L8)]ClO4, 2(CH2Cl2) | HUPLUA | CP2S3 | [43] |
| 69 | Ru(X1)(L8)Cl | IQUFEG | C4ClS2 | [66] |
| 70 | Ru(X1)(L29)Cl | IQUFIK | C4ClS2 | [66] |
| 71 | [Ru(X1)(L8)(CH3CN)]PF6 | IQUFOQ | C4NS2 | [66] |
| 72 | (p-cymene)Ru(L79)Cl | PEKVIN | C3ClS2 | [67] |
| Crystal | Formulation | REFCODE | Donor Set | Ref. |
|---|---|---|---|---|
| 73 | Tp*Rh(η2-L24)(η1-L24) | BILHIO | N3S3 | [68] |
| 74 | [Rh(H){κ3-P,Si,P′-SiPh(NCH2PPh2)2C6H4}(L24)], 0.5(C6H6) | WODHOO | HP2S2Si | [69] |
| 75 | Rh(phpy)2(L24) | EMOKAU | C2N2S2 | [70] |
| 76 | Rh(phpy)2(L66) | XOCFUS | C2N2S2 | [71] |
| 77 | (PMe3)2Rh(X2)(L24) | KUPVOH | C2P2S2 | [72] |
| Crystal | Formulation | REFCODE | Donor Set | Ref. |
|---|---|---|---|---|
| 78 | Pd(L4)2 | YIRSIE | S4 | [73] |
| 79 | Pd(L8)2 | OMUJEN01 * | S4 | [74,75,76] |
| 80 | Pd(L11)2 | ODOYAI | S4 | [77] |
| 81 | Pd(L13)2 | LIGSAW | S4 | [78] |
| 82 | Pd(L15)2 | ZOJVAX | S4 | [79] |
| 83 | Pd(L16)2 | FUHXEN | S4 | [80] |
| 84 | Pd(L24)2 | DETCPD02 | S4 | [81] |
| 85 | Pd(L25)2 | FEVCER | S4 | [82] |
| 86 | Pd(L30)2 | QOWXUW | S4 | [83] |
| 87 | Pd(L33)2 | EMOKOI | S4 | [84] |
| 88 | Pd(L35)2 | ZOJTUP | S4 | [79] |
| 89 | Pd(L39)2 | LIGSEA | S4 | [78] |
| 90 | Pd(L39)2, C5H5N | ZOJTID | S4 | [79] |
| 91 | Pd(L39)2, 2Cr(C6H6)2, 2(C60) | CEBCES | S4 | [85] |
| 92 | Pd(L41)2 | HOMXEO | S4 | [86] |
| 93 | Pd(L42)2 | LUKTES | S4 | [87] |
| 94 | Pd(L47)2, C5H5N | ZOJTOJ | S4 | [79] |
| 95 | Pd(L57)2 | UBIFUI | S4 | [88] |
| 96 | Pd(L63)2 | XAZZOO | S4 | [89] |
| 97 | Pd(L64)2 | JESJUN | S4 | [90] |
| 98 | [Pd(L66)2], 3H2O | BEZKAU | S4 | [91] |
| 99 | Pd2(L86)2 | KENYIN | S4 | [92] |
| 100 | Pd2(L86)2, CHCl3 | KENYOT | S4 | [92] |
| 101 | Pd2(L87)2, 2(CH3OH) | IXIWAN | S4 | [93] |
| 102 | [Pd2(L44)2]Cl2, 1.5(CH3OH), 2(H2O) | IGUBAP | P2S2 | [94] |
| 103 | (fcdpm)Pd(L24) | YEFVOW | N2S2 | [95] |
| 104 | (fcdpm)Pd(L29) | YEFVUC | N2S2 | [95] |
| 105 | (fcdpm)Pd(L43) | VIZJOF | N2S2 | [96] |
| 106 | [Pd(dmbp)(L75)][PF6]2 | XEDWOV | N2S2 | [97] |
| 107 | [Pd(bpy)(L75)][PF6]2, CH3CN | XEDWUB | N2S2 | [97] |
| 108 | [(dppe)Pd(L57)]Cl, 1.33(H2O), CHCl3 | DIFQIU | P2S2 | [26] |
| 109 | [(dppp)Pd(L24)]BF4, 2(CHCl3) | WUMQEB | P2S2 | [98] |
| 110 | [(dppf)Pd(L57)]PF6, CH2Cl2 | DOZKOV | P2S2 | [99] |
| 111 | {[(PPh3)2Pd]2(L74)}[PF6]2, (CH3CH2)2O | KEKKAP | P2S2 | [100] |
| 112 | {[(PPh3)2Pd]2(L74)}[PF6]2, (CH3CH2)2O | KEKKET | P2S2 | [100] |
| 113 | {[(PPh3)2Pd]2(L85)}[PF6]2, (CH3CH2)2O | KEKKIX | P2S2 | [100] |
| 114 | {[(dppf)Pd]2(L74)}[BF4]2, 2.6(CH2Cl2) | RUDLEI | P2S2 | [101] |
| 115 | {[(dppf)Pd]2(L85)}[PF6]2, CH2Cl2, CH3CH2OH | ISEWIN | P2S2 | [102] |
| 116 | (PPh3)Pd(L31)Cl | RASYAN | ClPS2 | [103] |
| 117 | (PPh3)Pd(L32)Cl | NAJVEC | ClPS2 | [104] |
| 118 | (PPh3)Pd(L47)Cl | KIJROL | ClPS2 | [105] |
| 119 | (PPh3)Pd(L57)Cl, 0.5(CHCl3) | DICXOE | ClPS2 | [26] |
| 120 | (PPh3)Pd(L64)Cl | SAYZOI | ClPS2 | [106] |
| 121 | (PPh3)Pd(L68)Cl, 0.5(CHCl3) | NAJNOE | ClPS2 | [104] |
| 122 | (PPh3)Pd(L81)Cl | BULBAM | ClPS2 | [107] |
| 123 | [P(o-tolyl)3]Pd(L81)Cl | BUKZUD * | ClPS2 | [107,108] |
| 124 | [P(p-tolyl)3]Pd(L39)Cl | VANWAL | ClPS2 | [109] |
| 125 | [P(p-tolyl)3]Pd(L69)Cl | ROHJUW | ClPS2 | [110] |
| 126 | [P(p-tolyl)3]Pd(L71)Cl, CHCl3 | UNOYAZ | ClPS2 | [111] |
| 127 | [P(p-tolyl)3]Pd(L76)Cl | ROHKAD | ClPS2 | [110] |
| 128 | [P(C6H4Cl-p)3]Pd(L69)Cl | ADUHAL | ClPS2 | [112] |
| 129 | [P(C6H4Cl-p)3]Pd(L72)Cl | MEHHIT | ClPS2 | [113] |
| 130 | [P(C6H4Cl-p)3]Pd(L77)Cl | QEKTAE | ClPS2 | [114] |
| 131 | [P(C6H4F-p)]Pd(L69)Cl | ADUGOY | ClPS2 | [112] |
| 132 | [P(C6H4F-p)]Pd(L71)Cl | ADUGUE | ClPS2 | [112] |
| 133 | [P(C6H4F-p)]Pd(L72)Cl | MEHHEP | ClPS2 | [113] |
| 134 | [P(C6H4F-p)]Pd(L77)Cl} 0.5(CH3COCH3) | QEKSUX | ClPS2 | [114] |
| 135 | [PPh2(o-tolyl)]Pd(L71)Cl | GIQGUK | ClPS2 | [115] |
| 136 | [PPh2(p-tolyl)]Pd(L31)Cl | RASYER | ClPS2 | [103] |
| 137 | [PPh2(p-tolyl)]Pd(L39)Cl | VANVUE | ClPS2 | [109] |
| 138 | [PPh2(p-tolyl)]Pd(L67)Cl | VANVOY | ClPS2 | [109] |
| 139 | [PPh2(p-tolyl)]Pd(L69)Cl | ROHJOQ | ClPS2 | [110] |
| 140 | [PPh2(p-tolyl)]Pd(L71)Cl | UNOYON | ClPS2 | [111] |
| 141 | [PPh2(C6H4OMe-o)]Pd(L24)Cl, H2O | RAFLER | ClPS2 | [116] |
| 142 | [PPh2(nPr)]Pd(L26)Cl | EHOBOU | ClPS2 | [117] |
| 143 | [PPh2(tBu)]Pd(L38)Cl | EHOBUA | ClPS2 | [117] |
| 144 | [PPh2(CH2Ph)]Pd(L8)Cl | RAFLAN | ClPS2 | [116] |
| 145 | [PPh2(CH2Ph)]Pd(L24)Cl | GIQHAR | ClPS2 | [115] |
| 146 | [(PPh3)Pd(L68)(SCN)], 0.5(CH2Cl2) | NAJNIY | PS3 | [104] |
| 147 | [tBu(Ph)POH][tBu(Ph)P(=O)]Pd(L24) | SAGBIM | P2S2 | [118] |
| 148 | Cu{[tBu(PhP)(O)]Pd(L24)}2 | SAFZUV | P2S2 | [118] |
| 149 | Pd(phpz)(L24) | UMEBEU | CNS2 | [119] |
| 150 | Pd(phpy)(L63) | JUPNIT | CNS2 | [120] |
| 151 | Pd(C^N)(L24) | OVEPOV | CNS2 | [121] |
| 152 | Pd(X3)(L24), CH3(CH2)4CH3, 0.5(CH2Cl2) | FOHYEH | CNS2 | [122] |
| 153 | Pd(κ2-C,N-CH2SiPh2(CH2NC5H10))(L24) | EZEXIQ | CNS2 | [123] |
| 154 | (PPh3)Pd(pyr)(L57) | UQANEG | CPS2 | [124] |
| 155 | Pd(η3-C4H7)(L8) | ODOXOV | C2S2 | [77] |
| 156 | Pd(η3-C4H7)(L28) | ODOYEM | C2S2 | [77] |
| Crystal | Formulation | REFCODE | Donor Set | Ref. |
|---|---|---|---|---|
| 157 | Os(L8)2(CO)2 | KAZHIE | C2S4 | [130] |
| 158 | (PPh3)2Os(SiCl3)(L8)(CO), 2(CH2Cl2) | NEKGOA | CP2S2Si | [125] |
| 159 | (PPh3)2Os(SiMeCl2)(L8)(CO), 2(CH2Cl2) | NEKGIU | CP2S2Si | [125] |
| 160 | (PPh3)2Os(SiMe2Cl)(L8)(CO), CH2Cl2 | FIXRAG | CP2S2Si | [126] |
| 161 | (PPh3)2Os(SiMe2OH)(L8)(CO), x(CH3(CH2)5CH3) | FIXREK | CP2S2Si | [126] |
| 162 | (PPh3)2Os(SiMe2OSiMe3)(L8)(CO) | FIXROU | CP2S2Si | [126] |
| 163 | (PPh3)2Os(SiOH3)(L8)(CO) | FIXRIO | CP2S2Si | [126] |
| 164 | (PPh3)2Os(SnCl3)(L8)(CO), CHCl3 | QEWPAK | CP2S2Sn | [127] |
| 165 | (PPh3)2Os(SnF3)(L8)(CO), 2(CH2Cl2) | QEWPEO | CP2S2Sn | [127] |
| 166 | (PPh3)2Os(SnH3)(L8)(CO), C6H6 | QEWNUC | CP2S2Sn | [127] |
| 167 | (PPh3)2Os(SnHMe2)(L8)(CO), C6H6 | QEWPUE | CP2S2Sn | [127] |
| 168 | (PPh3)2Os(SnMeF2)(L8)(CO), CH2Cl2 | QEWPOY | CP2S2Sn | [127] |
| 169 | (PPh3)2Os(SnMe2Cl)(L8)(CO), C6H6 | TAXYOH | CP2S2Sn | [128] |
| 170 | (PPh3)2Os(SnMe2SnPh3)(L8)(CO), 2(CH2Cl2) | PENLIE | CP2S2Sn | [129] |
| 171 | (PPh3)Os(SnMe2C6H4PPh2)(L8)(CO) | TAXYUN | CP2S2Sn | [128] |
| 172 | (PPh3)Os(SnMeClC6H4PPh2)(L8)(CO) | TAXZAU | CP2S2Sn | [128] |
| 173 | Os3(L8)2(CO)10 | KUJFED | C3OsS2 | [131] |
| 174 | [Os4(L8)2(CO)12(CO2)(S)], 2(CH2Cl2) | KUJFIH | C3OS2, C3S3 | [131] |
| 175 | Os3(H)(L8)(CO)9 | KUJFUT | C3Os2S/C3HOs2S | [131] |
| 176 | Os3(H)(L24)(CO)9 | SATGUQ | C3Os2S/C3HOs2S | [132] |
| Crystal | Formulation | REFCODE | Donor Set | Ref. |
|---|---|---|---|---|
| 177 | Ir(ppy)2(L24) | WIHPUZ | C2N2S2 | [133] |
| 178 | Ir(buppy)2(L24) | MADKAE | C2N2S2 | [134] |
| 179 | Ir(pba)2(L24) | QOFTEN | C2N2S2 | [135] |
| 180 | Ir(tfmppy)2(L29) | FODDUA | C2N2S2 | [136] |
| 181 | Ir(btfmppy)2(L29) | FODDOU | C2N2S2 | [136] |
| 182 | Ir(tfmbpy)2(L29) | FODFAI | C2N2S2 | [136] |
| 183 | Ir(tfmpiq)2(L29) | HOFCUD | C2N2S2 | [137] |
| 184 | Ir(tfmpiq)2(L46), unknown solvate | HOFDAK | C2N2S2 | [137] |
| 185 | Ir(tfmpiq)2(L83), unknown solvate | HOFDEO | C2N2S2 | [137] |
| 186 | Ir(pqz)2(L82) | ZUQLUU | C2N2S2 | [27] |
| 187 | Ir(tfmpqz)2(L29) | LIYWUO | C2N2S2 | [138] |
| 188 | Ir(tfmpqz)2(L46) | CUMGUP | C2N2S2 | [139] |
| 189 | Ir(tfmpqz)2(L48) | CUMGOJ | C2N2S2 | [139] |
| 190 | Ir(tfmpqz)2(L49) | CUMHOK | C2N2S2 | [139] |
| 191 | Ir(tfmpqz)2(L50) | VIWDAJ | C2N2S2 | [140] |
| 192 | Ir(tfmpqz)2(L82) | LIYWOI | C2N2S2 | [138] |
| 193 | Ir(tfmpqz)2(L83) | VIWCUC | C2N2S2 | [140] |
| 194 | Ir(dpp)2(L24), CH2Cl2, unknown solvate | IQAFEN | C2N2S2 | [141] |
| 195 | Ir(dpci)2(L82), CH2Cl2, 1.5(H2O) | VITHEN | C2N2S2 | [142] |
| 196 | Ir(pbt)2(L24) | IXOJEM * | C2N2S2 | [143,144] |
| 197 | Ir(ptbt)2(L24) | WOTQUU | C2N2S2 | [145] |
| 198 | Ir(X4)2(L24), 0.5(H2O) | WEKROU * | C2N2S2 | [146,147] |
| 199 | (PPh3)2Ir(C5H5SMe)(L24) | ANOKAQ | C2P2S2 | [148] |
| 200 | [(PPh3)2Ir(C5H4SMe)(L24)]PF6 | ANOJUJ | C2P2S2 | [148] |
| 201 | [(PPh3)2Ir(C5H3Me-3SMe)(L24)]PF6, ClCH2CH2Cl | ANOKEU | C2P2S2 | [148] |
| 202 | [(PPh3)2Ir(C5H3OEt-3SMe)(L24)]PF6 | ANOKIY | C2P2S2 | [148] |
| 203 | [Cp*Ir(L24)(CO)]CF3SO3 | GEVGUK | C4S2 | [149] |
| 204 | [Cp*Ir(L24)]2[ClO4]2 | WIHNIL | C3S3 | [150] |
| 205 | Cp*Ir(mtz)(L8), 5-methyltetrazole | NOJPUY | C3NS2 | [151] |
| 206 | [{Cp*Ir(L8)}2(mtz)]PF6 | NOJQAF | C3NS2 | [151] |
| 207 | Cp*Ir(SH)(PMe3)(L5), CH2Cl2 | SIKPUY | C3PS2 | [152] |
| 208 | Cp*Ir(H)(PMe3)(L5) | SIKQEJ | C3HPS | [152] |
| 209 | [Cp*Ir(PPh3)(L24)]3[Cp*Ir(PPh3)(L25)(NCH3)][I]4 | OYUJEY | C3NPS | [153] |
| Crystal | Formulation | REFCODE | Donor Set | Ref. |
|---|---|---|---|---|
| 210 | Pt(L4)2 | YIRSOK | S4 | [73] |
| 211 | Pt(L6)2 | YIRXEF | S4 | [73] |
| 212 | Pt(L8)2 | TEDRIG * | S4 | [75,76] |
| 213 | Pt(L16)2 | FUHXIR | S4 | [80] |
| 214 | Pt(L27)2 | TUCMAH | S4 | [154] |
| 215 | Pt(L29)2 | LOWJEP | S4 | [155] |
| 216 | [Pt(L31)2], C60 | MOKNOR | S4 | [156] |
| 217 | Pt(L33)2 | EMOKUO | S4 | [84] |
| 218 | Pt(L34)2 | ZOJHUE | S4 | [155,157] |
| 219 | [Pt(L39)2], C6H5CN | ZOJJAM | S4 | [157] |
| 220 | Pt(L41)2 | LASPIH | S4 | [158] |
| 221 | Pt(L45)2 | UHOGIJ | S4 | [159] |
| 222 | Pt(L52)2 | MOHNUU | S4 | [160] |
| 223 | [Pt(L52)2], 6((CH3)2SO) | GUCQAZ | S4 | [161] |
| 224 | Pt(L54)2 | HOMXAK | S4 | [86] |
| 225 | Pt(L55)2, unknown solvate | GUMCEZ | S4 | [162] |
| 226 | Pt(L70)2 | WIFVUF | S4 | [163] |
| 227 | Pt(L80)2 | CAJWOC | S4 | [164] |
| 228 | Pt(L62)2 | GEZHEA | S3N | [165] |
| 229 | [Zn(H2O)4Pt(L17)2]n, 4(H2O) | GUCQED | S4 | [161] |
| 230 | [Zn(H2O)4Pt(L58)2]n, 2(H2O) | GUCQIH | S4 | [161] |
| 231 | {Zn(H2O)3Pt(L53)2]n, 3(H2O) | GUCQUT | S4 | [161] |
| 232 | {[Zn(H2O)4]2Pt(L51)2}n, 6(H2O) | GUCQON | S4 | [161] |
| 233 | [Pt(L57)Cl]5, 2(ClCH2CH2Cl) | HACCAR | ClS3 | [166] |
| 234 | (PPh3)Pt(η1-L57)(η2-L57), 2(CH2Cl2), 0.25(CH3(CH2)3CH3) | DIFQOA | PS3 | [26] |
| 235 | {[(PEt3)2Pt]2(L74)}(PF6)2, unknown solvate | RUDLAE | P2S2 | [101] |
| 236 | {[(PPh3)2Pt]2(L74)}(PF6)2 | RUDKUX | P2S2 | [101] |
| 237 | Pt(L57)(Cl)((CH3)2SO) | NASDAO | ClS3 | [167] |
| 238 | (PEt3)Pt(L24)Cl | TOWNUR | ClPS2 | [168] |
| 239 | (PPh3)Pt(L57)Cl, 0.5(CH2Cl2) | HACBUK | ClPS2 | [166] |
| 240 | [P(C6H4Cl-p)3]Pt(L73)Cl | AZIDAQ | ClPS2 | [169] |
| 241 | [P(C6H4F-p)3]Pt(L73)Cl | AZIDEU | ClPS2 | [169] |
| 242 | [P(C6H4F-p)3]Pt(L78)Cl | AZICUJ | ClPS2 | [169] |
| 243 | [P(p-tolyl)3]Pt(L76)Cl | RIZNEW | ClPS2 | [170] |
| 244 | Pt(bmi)(L24)I | AHADAR | CIS2 | [171] |
| 245 | Pt(bzq)(L8) | EQONOO | CNS2 | [172] |
| 246 | Pt(bzq)(L57) | EQONII | CNS2 | [172] |
| 247 | Pt(X5)(L24) | PUPHUF | CNS2 | [173] |
| 248 | [Pt(L24)]2(bpy), CH2Cl2 | RIZBUA | CNS2 | [174] |
| 249 | (PEt3)Pt(L24)(CH2(iPr)) | HUWGIR | CPS2 | [175] |
| 250 | Pt(PPh3)(L24)C≡W(CO)2(Tp*) | JUQRAR | CPS2 | [176] |
| 251 | Pt(bzq)(L8)(PPh2)2Pt(C6F5)2, 2(CH2Cl2) | QUFXAT | CNP2S2 | [177] |
| 252 | {Ag[Pt(bzq)(L57)]2}n, n(ClO4), n(CH3CN) | EQOPEG | AgCNS2 | [172] |
| Metal Ion | Mean Shift, ppm | Sample Standard Deviation, ppm |
|---|---|---|
| Ru(II) | 3.20 | 0.50 |
| Ru(III) | 3.63 | 0.37 |
| Os(II) | 2.47 | 0.63 |
| Rh(III) | 3.63 | 0.37 |
| Ir(III) | 3.29 | 0.44 |
| Pd(II) | 3.82 | 0.61 |
| Pt(II) | 3.83 | 0.50 |
| Pt(IV) | 3.51 | 0.26 |
| Complex | δ, ppm | Ref. |
|---|---|---|
| [Ru(L8)3] | 28.85 | [25] |
| 28.70 | [32] | |
| [Ru(L57)3] | 45.03, 36.28 | [25] |
| 44.40, 36.00 | [32] | |
| [Ru(L19)3] | 26.80, 26.70, 21.83, 14.63 | [25] |
| [Ru(L20)3] | 26.70, 26.23, 21.92, 14.55 | [25] |
| [Ru(L21)3] | 26.82, 26.43, 21.32, 14.40 | [25] |
| α-[Ru2(L8)5]Cl | 3.55, 3.52, 3.50, 3.20, 2.99 | [25] |
| 3.62, 3.46, 3.47, 3.17, 2.93 | [32] | |
| β-[Ru2(L8)5]Cl | 3.55, 3.54, 3.51, 3.20, 3.19 | [32] |
| α-[Ru2(L57)5]Cl | 4.05–3.37 | [25] |
| 4.25–3.15 | [32] | |
| β-[Ru2(L57)5]Cl | 3.98–3.40 | [32] |
| α-[Ru2(L19)5]Cl | 4.60–4.00, 3.48–3.14 | [25] |
| β-[Ru2(L20)5]Cl | 5.14–4.45, 3.71–3.04 | [25] |
| α-[Ru2(L21)5]Cl | 4.72–4.01, 3.64–2.62 | [25] |
| Complex | ΔG, kJ mol−1 | ν(C–N), cm−1 | Ref. |
|---|---|---|---|
| [Ru(CO)2(L8)2] | 78 ± 1 | Not reported | [182] |
| [Ru(CO)(PEt3)(L8)2] | 74 ± 1 and 71 ± 1 | Not reported | [46] |
| [Os(CO)2(L8)2] | 74.2 ± 0.5 | Not reported | [130] |
| [{PtMe3(L1)}2] | 71.43 ± 0.14 | 1609 | [178] |
| [{PtMe3(L8)}2] | 81.54 ± 0.19 | 1530 | [178] |
| [{PtMe3(L24)}2] | 82.89 ± 0.56 | 1516 | [178] |
| [{PtMe3(L2)}2] | 72.27 ± 0.05 | 1517 | [178] |
| [{PtMe3(L3}2] | 66.36 ± 0.05 | 1518 | [178] |
| [{PtMe3(L4)}2] | 65.61 ± 0.01 | 1527 | [178] |
| [{PtMe3(L14)}2] | 86.54 ± 0.29 87.24 ± 0.27 87.15 ± 0.35 | 1489 | [178] |
| Complex | δ, ppm a | δ, ppm b | Ref. |
|---|---|---|---|
| [Pt(pbt)(L24)] | 686 | - | [143] |
| [Pt(L18) 2] | 626 | - | [161] |
| [Pt(L59)2] | 720 | - | [161] |
| Zn[Pt(L58)2] | 793 | - | [161] |
| [Pt(L52)2] | 603 | - | [161] |
| Zn[Pt(L53)2] | 614 | - | [161] |
| Zn2[Pt(L51)2] | 678 | - | [161] |
| [WPt(μ-C)(L24)(CO)2(PPh3)(Tp*)] | 694 | - | [176] |
| [Pt(pmea)(L24)] | 810 | - | [173] |
| [PtMeI(pmea)(L24)] | 1333 | - | [173] |
| [{PtMe3(L1)}2] | 1416 | - | [178] |
| [{PtMe3(L8)}2] | 1577 | - | [178] |
| [{PtMe3(L24)}2] | 1564 | - | [178] |
| [{PtMe3(L2)}2] | 1623, 1609, 1598, 1587 | - | [178] |
| [{PtMe3(L3}2] | 1626, 1609, 1607, 1596 | - | [178] |
| [{PtMe3(L4}2] | 1632, 1600, 1587, 1582 | - | [178] |
| [{PtMe3(L14)}2] | 1584, 1575, 1569, 1559 | 1601 | [178] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.S.; Yeo, C.I.; Tiekink, E.R.T.; Heard, P.J. Dithiocarbamate Complexes of Platinum Group Metals: Structural Aspects and Applications. Inorganics 2021, 9, 60. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9080060
Tan YS, Yeo CI, Tiekink ERT, Heard PJ. Dithiocarbamate Complexes of Platinum Group Metals: Structural Aspects and Applications. Inorganics. 2021; 9(8):60. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9080060
Chicago/Turabian StyleTan, Yee Seng, Chien Ing Yeo, Edward R. T. Tiekink, and Peter J. Heard. 2021. "Dithiocarbamate Complexes of Platinum Group Metals: Structural Aspects and Applications" Inorganics 9, no. 8: 60. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9080060