Copper(II) Prevents the Saccarine-Dialkylcyanamide Coupling by Forming Mononuclear (Saccharinate)(Dialkylcyanamide)copper(II) Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
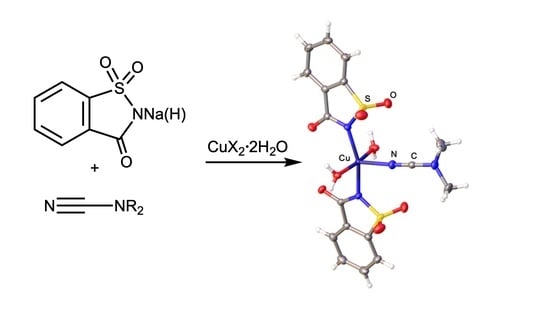
2.2. X-ray Diffraction Studies
2.3. Theoretical Calculations
3. Materials and Methods
3.1. Synthetic Work
3.2. X-ray Structure Determinations
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martyn, D.; Darch, M.; Roberts, A.; Lee, H.Y.; Yaqiong Tian, T.; Kaburagi, N.; Belmar, P. Low-/No-Calorie Sweeteners: A Review of Global Intakes. Nutrients 2018, 10, 357. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Srivastava, S. Artificial sugar saccharin and its derivatives: Role as a catalyst. RSC Adv. 2020, 10, 36571–36608. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F.; Ahmad, S.; Naqvi, S.A.R.; Khan, S.G.; Suleman, M. Update on the Reactivity of Saccharin: An Excellent Precursor for the Synthesis of Biologically Important Molecules. Heterocycles 2017, 94, 1389–1426. [Google Scholar]
- Figueroa, F.N.; Heredia, A.A.; Peñéñory, A.B.; Sampedro, D.; Argüello, J.E.; Oksdath-Mansilla, G. Regioselective Photocycloaddition of Saccharin Anion to π-Systems: Continuous-Flow Synthesis of Benzosultams. J. Org. Chem. 2019, 84, 3871–3880. [Google Scholar] [CrossRef]
- Zaharani, L.; Ghaffari Khaligh, N.; Shahnavaz, Z.; Rafie Johan, M. Arene diazonium saccharin intermediates: A greener and cost-effective alternative method for the preparation of aryl iodide. Turk. J. Chem. 2020, 44, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bubun, B.; Vaishali, B.; Amninder, K.; Gurpreet, K.; Arvind, S. Catalytic Applications of Saccharin and its Derivatives in Organic Synthesis. Curr. Org. Chem. 2019, 23, 3191–3205. [Google Scholar]
- Thakuria, R.; Delori, A.; Jones, W.; Lipert, M.P.; Roy, L.; Rodríguez-Hornedo, N. Pharmaceutical cocrystals and poorly soluble drugs. Int. J. Pharm. 2013, 453, 101–125. [Google Scholar] [CrossRef] [PubMed]
- Dubovtsev, A.Y.; Ivanov, D.M.; Dabranskaya, U.; Bokach, N.A.; Kukushkin, V.Y. Saccharin guanidination via facile three-component “two saccharins-one dialkylcyanamide” integration. New J. Chem. 2019, 43, 10685–10688. [Google Scholar] [CrossRef]
- Abramovitch, R.A.; Ooi, G.H.C.; Sun, H.-L.; Pierrot, M.; Baldy, A.; Estienne, J. The reaction of saccharin derivatives with N,N-didethylprop-1-ynamine; formation of cyclobutenyl saccharinates and of a spiro-oxete. J. Chem. Soc. Chem. Commun. 1984, 23, 1583–1584. [Google Scholar] [CrossRef]
- Habibi-Khorassani, S.M.; Shahraki, M.; Darijani, M. Structural effects on kinetics and a mechanistic investigation of the reaction between DMAD and N-H heterocyclic compound in the presence of triphenylarsine: Spectrophotometry approach. Chem. Central J. 2017, 11, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, E.; Nespital, V.; Beutler, R. Zur reaktivität des knallsäureamids umsetzung von saccharin bzw. thiosaccharin mit N-isocyanaminen. Tetrahedron Lett. 1971, 12, 525–528. [Google Scholar] [CrossRef]
- Tan, D.; Friščić, T. Carbodiimide insertion into sulfonimides: One-step route to azepine derivatives via a two-atom saccharin ring expansion. Chem. Commun. 2017, 53, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, J.G.; Lerch, U. Carbodiimide-sulfoxide reactions. XII. Reactions of sulfonamides. J. Org. Chem. 1971, 36, 3686–3691. [Google Scholar] [CrossRef]
- Bokach, N.A.; Kukushkin, V.Y. Coordination chemistry of dialkylcyanamides: Binding properties, synthesis of metal complexes, and ligand reactivity. Coord. Chem. Rev. 2013, 257, 2293–2316. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Rassadin, V.A.; Bokach, N.A.; Kukushkin, V.Y. Metal-involving generation of aminoheterocycles from N-substituted cyanamides: Toward sustainable chemistry (a Minireview). Inorg. Chim. Acta 2017, 455, 446–454. [Google Scholar] [CrossRef]
- Turner, D.R.; Chesman, A.S.R.; Murray, K.S.; Deacon, G.B.; Batten, S.R. The chemistry and complexes of small cyano anions. Chem. Commun. 2011, 47, 10189–10210. [Google Scholar] [CrossRef]
- Batten, S.R.; Murray, K.S. Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide. Coord. Chem. Rev. 2003, 246, 103–130. [Google Scholar] [CrossRef]
- He, G.; Chow, P.S.; Tan, R.B.H. Predicting Multicomponent Crystal Formation: The Interplay between Homomeric and Heteromeric Interactions. Cryst. Growth Des. 2009, 9, 4529–4532. [Google Scholar] [CrossRef]
- Haider, S.Z.; Malik, K.M.A.; Ahmed, K.J.; Hess, H.; Riffel, H.; Hursthouse, M.B. X-ray crystal structures of metal saccharin complexes of general formula [M(C7H4NO3S)2(H2O)4]·2H2O, where M = Fe(II), Co(II), Ni(II) and Cu(II). Inorg. Chim. Acta 1983, 72, 21–27. [Google Scholar] [CrossRef]
- Melekhova, A.A.; Novikov, A.S.; Panikorovskii, T.L.; Bokach, N.A.; Kukushkin, V.Y. A novel family of homoleptic copper(I) complexes featuring disubstituted cyanamides: A combined synthetic, structural, and theoretical study. New J. Chem. 2017, 41, 14557–14566. [Google Scholar] [CrossRef] [Green Version]
- Melekhova, A.A.; Novikov, A.S.; Dubovtsev, A.Y.; Zolotarev, A.A.; Bokach, N.A. Tris(3,5-dimethylpyrazolyl)methane copper(I) complexes featuring one disubstituted cyanamide ligand. Inorg. Chim. Acta 2019, 484, 69–74. [Google Scholar] [CrossRef]
- Toikka, Y.N.; Mikherdov, A.S.; Ivanov, D.M.; Mooibroek, T.J.; Bokach, N.A.; Kukushkin, V.Y. Cyanamides as π-Hole Donor Components of Structure-Directing (Cyanamide)···Arene Noncovalent Interactions. Cryst. Growth Des. 2020, 20, 4783–4793. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Çakir, S.; Bulut, I.; Aoki, K. Crystal structures of mixed ligand complexes containing saccharinate and nicotinamide: [Cu(saccharinato)2 (nicotinamide) (H2O)]⋅(H2O) and [M(nicotinamide)2 (H2O)4]⋅(saccharinate)2 (M = Ni2+, Co2+). J. Chem. Crystallogr. 2003, 33, 875–884. [Google Scholar] [CrossRef]
- Williams, P.A.M.; Ferrer, E.G.; Pasquevich, K.A.; Baran, E.J.; Chaia, Z.; Castellano, E.E.; Piro, O.E. Characterization of Two New Copper(II) Complexes with Saccharinate and Benzimidazole as Ligands. Zeits. Anorg. Allg. Chem. 2000, 626, 2509–2514. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Senel, E.; Kazak, C. Concomitant Crystallization of Copper(II) Sachharinato Complexes with 2-methylpyrazine as a Monomer and an One-dimensional Polymer: Syntheses, Crystal Structures, Spectroscopic and Thermal Properties. J. Inorg. Organomet. Polym. Mater. 2008, 18, 407–413. [Google Scholar] [CrossRef]
- Goreshnik, E.A.; Oliinik, V.V. Tetranuclear Copper(II) Complex Cu4OCl6. 4(C3H5)2NCN: Synthesis, Structure, and Magnetic Properties. Russ. J. Inorg. Chem. (Zhurnal Neorg. Khimii) 1996, 41, 195–197. [Google Scholar]
- Kinzhalov, M.A.; Parfenova, S.N.; Novikov, A.S.; Katlenok, E.A.; Puzyk, M.V.; Avdontceva, M.S.; Bokach, N.A. Cyclometalated Iridium(III) Complexes Featuring Disubstituted Cyanamides. Chemistryselect 2018, 3, 11875–11880. [Google Scholar] [CrossRef]
- Smirnov, A.S.; Butukhanova, E.S.; Bokach, N.A.; Starova, G.L.; Gurzhiy, V.V.; Kuznetsov, M.L.; Kukushkin, V.Y. Novel (cyanamide)Zn-II complexes and zinc(II)-mediated hydration of the cyanamide ligandst. Dalton Trans. 2014, 43, 15798–15811. [Google Scholar] [CrossRef]
- Tom Dieck, H.; Brehm, H.P. Vierkernige Komplexe mit trigonal-bipyramidal koordiniertem Kupfer(II), II. Synthese und Substitution an der axialen Position. Chem. Ber. 1969, 102, 3577–3583. [Google Scholar] [CrossRef]
- Kuang, S.M.; Cuttell, D.G.; McMillin, D.R.; Fanwick, P.E.; Walton, R.A. Synthesis and Structural Characterization of Cu(I) and Ni(II) Complexes that Contain the Bis[2-(diphenylphosphino)phenyl]ether Ligand. Novel Emission Properties for the Cu(I) Species. Inorg. Chem. 2002, 41, 3313–3322. [Google Scholar]
- Bera, J.K.; Nethaji, M.; Samuelson, A.G. Anion-Controlled Nuclearity and Metal−Metal Distances in Copper(I)−dppm Complexes (dppm = Bis(diphenylphosphino)methane). Inorg. Chem. 1999, 38, 218–228. [Google Scholar]
- Olijnik, V.V.; Goreshnik, E.A.; Myskiv, M.G.; Pecharskii, V.K. Copper(I) nitrate π-complexes. Synthesis and crystal structure of CuNo3(CH2=CH-CH2)2NCN. J. Struct. Chem. 1994, 35, 87–90. [Google Scholar] [CrossRef]
- Baran, E.J.; Yilmaz, V.T. Metal complexes of saccharin. Coord. Chem. Rev. 2006, 250, 1980–1999. [Google Scholar] [CrossRef]
- Uçar, İ.; Bozkurt, E.; Kazak, C.; Bulut, A. Structural characterization and EPR spectral studies on mononuclear copper(II) complex of saccharin with ethylnicotinate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 72, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Naumov, P.; Jovanovski, G.; Drew, M.G.B.; Ng, S.W. Outer-sphere coordination, N-coordination and O-coordination of the deprotonated saccharin in copper(II) saccharinato complexes. Implications for the saccharinato carbonyl stretching frequency. Inorg. Chim. Acta 2001, 314, 154–162. [Google Scholar] [CrossRef]
- Naumov, P.; Jovanovski, G.; Ristova, M.; Razak, I.A.; Çkir, S.; Chantrapromma, S.; Fun, H.-K.; Ng, S.W. Coordination of Deprotonated Saccharin in Copper(II) Complexes. Structural Role of the Saccharinate Directed by the Ancillary N-heterocyclic Ligands. Zeits. Anorg. Allg. Chem. 2002, 628, 2930–2939. [Google Scholar] [CrossRef]
- Yilmaz, V.T.; Senel, E.; Kazak, C. Influence of the Crystallization Solvent on the Molecular Structures of Copper(II) Saccharinato Complexes with Pyridazine: Synthesis, X-ray Crystallography, Spectroscopy, Photoluminescence, and Thermal Properties. Austr. J. Chem. 2008, 61, 634–639. [Google Scholar] [CrossRef]
- Jovanovski, G.; Naumov, P.; Grupče, O.; Kaitner, B. Structural study of monoaquabis(pyridine)bis(saccharinato)copper(II), [Cu(H2O)(py)2(sac)2]. Eur. J. Solid State Inorg. Chem. 1998, 35, 231–242. [Google Scholar] [CrossRef]
- Al-Jibori, S.A.; Al-Jibori, A.R.; Mohamad, H.A.; Al-Janabi, A.S.; Wagner, C.; Hogarth, G. Synthesis and reactivity towards amines of benzisothiazolinate-bridged paddlewheel dimers [M2(μ-bit)4·2H2O] (M = Mn, Co, Ni, Cu). Inorg. Chim. Acta 2019, 488, 152–158. [Google Scholar] [CrossRef]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Supplement. Tables of bond lengths determined by X-ray and neutron diffraction. Part 2. Organometallic compounds and co-ordination complexes of the d- and f-block metals. J. Chem. Soc. Dalton Trans. 1989, 12, S1–S83. [Google Scholar] [CrossRef]
- Alfaro, N.M.; Cotton, F.A.; Daniels, L.M.; Murillo, C.A. Mononuclear-dinuclear equilibrium for the pyridine adducts of chromium(II) saccharinates. Inorg. Chem. 1992, 31, 2718–2723. [Google Scholar] [CrossRef]
- Cotton, F.A.; Falvello, L.R.; Schwotzer, W.; Murillo, C.A.; Valle-Bourrouet, G. Two chromium(II) complexes with amidato-like ligands: A compound with the longest Cr☐Cr bond and a mononuclear compound with D2d symmetry. Inorg. Chim. Acta 1991, 190, 89–95. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Dapprich, S.; Frenking, G. Investigation of Donor-Acceptor Interactions: A Charge Decomposition Analysis Using Fragment Molecular Orbitals. J. Phys. Chem. 1995, 99, 9352–9362. [Google Scholar] [CrossRef]
- Novikov, A.S.; Kuznetsov, M.L. Theoretical study of Re(IV) and Ru(II) bis-isocyanide complexes and their reactivity in cycloaddition reactions with nitrones. Inorg. Chim. Acta 2012, 380, 78–89. [Google Scholar] [CrossRef]
- Novikov, A.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Theory of the Formation and Decomposition of N-Heterocyclic Aminooxycarbenes through Metal-Assisted [2+3]-Dipolar Cycloaddition/Retro-Cycloaddition. Chem. Eur. J. 2013, 19, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Melekhova, A.A.; Novikov, A.S.; Luzyanin, K.V.; Bokach, N.A.; Starova, G.L.; Gurzhiy, V.V.; Kukushkin, V.Y. Tris-isocyanide copper(I) complexes: Synthetic, structural, and theoretical study. Inorg. Chim. Acta 2015, 434, 31–36. [Google Scholar] [CrossRef]
- Melekhova, A.A.; Novikov, A.S.; Bokach, N.A.; Avdonceva, M.S.; Kukushkin, V.Y. Characterization of Cu-ligand bonds in tris-pyrazolylmethane isocyanide copper(I) complexes based upon combined X-ray diffraction and theoretical study. Inorg. Chim. Acta 2016, 450, 140–145. [Google Scholar] [CrossRef]
- Seifert, T.; Malo, M.; Kokkola, T.; Stéen, E.J.L.; Meinander, K.; Wallén, E.A.A.; Jarho, E.M.; Luthman, K. A scaffold replacement approach towards new sirtuin 2 inhibitors. Bioorg. Med. Chem. 2020, 28, 115231. [Google Scholar] [CrossRef]
- NETZSCH Proteus Software; v.6.1; Netzsch-Gerätebau: Bayern, Germany, 2013.
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlisPro: Single Crystal X-ray Diffraction Software. Available online: https://www.rigaku.com/products/crystallography/crysalis#specs (accessed on 7 September 2021).
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Figgen, D.; Rauhut, G.; Dolg, M.; Stoll, H. Energy-consistent pseudopotentials for group 11 and 12 atoms: Adjustment to multi-configuration Dirac–Hartree–Fock data. Chem. Phys. 2005, 311, 227–244. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toikka, Y.N.; Spiridonova, D.V.; Novikov, A.S.; Bokach, N.A. Copper(II) Prevents the Saccarine-Dialkylcyanamide Coupling by Forming Mononuclear (Saccharinate)(Dialkylcyanamide)copper(II) Complexes. Inorganics 2021, 9, 69. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9090069
Toikka YN, Spiridonova DV, Novikov AS, Bokach NA. Copper(II) Prevents the Saccarine-Dialkylcyanamide Coupling by Forming Mononuclear (Saccharinate)(Dialkylcyanamide)copper(II) Complexes. Inorganics. 2021; 9(9):69. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9090069
Chicago/Turabian StyleToikka, Yulia N., Dar’ya V. Spiridonova, Alexander S. Novikov, and Nadezhda A. Bokach. 2021. "Copper(II) Prevents the Saccarine-Dialkylcyanamide Coupling by Forming Mononuclear (Saccharinate)(Dialkylcyanamide)copper(II) Complexes" Inorganics 9, no. 9: 69. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics9090069