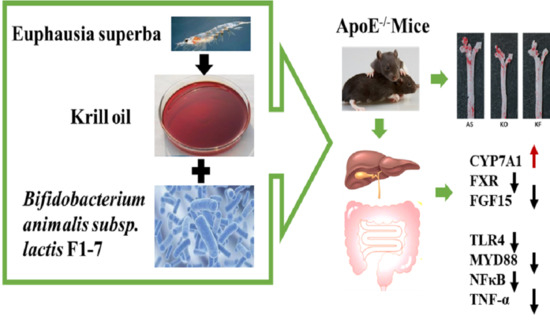
Krill Oil Combined with Bifidobacterium animalis subsp. lactis F1-7 Alleviates the Atherosclerosis of ApoE−/− Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. KO Samples and Composition Analysis
2.2. Strains and Culture
2.3. Animal and Experimental Design
2.4. Aortic Oil Red O Staining
2.5. Histological and Immunohistochemical Analysis
2.6. Biochemical Analysis
2.7. Determination of Total Bile Acids in Feces
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results
3.1. The Compositions Analysis of Krill Oil
3.2. The KO Combined with Bif. animalis F1-7 Reduced the Formation of Atherosclerotic Lesion in Apoe−/− Mice
3.3. The KO Combined with Bif. animalis F1-7 Reduced the Lipid Accumulation in Liver
3.4. The KO Combined with Bif. animalis F1-7 Alleviated the Serum Lipid Levels in Atherosclerotic Mice
3.5. Effects of the KO Combined with Bif. animalis F1-7 on the Total Bile Acids Level in Feces
3.6. Effects of KO Combined with Bif. animalis F1-7 on the Expression of Key Genes in Lipid Metabolism
3.7. The KO Combined with Bif. animalis F1-7 Reduced the Inflammatory Response in Serum of Atherosclerosis Mice
3.8. Effects of KO Combined with Bif. animalis F1-7 on the Targets Associated with Inflammatory Responses to Aortic Sinus
3.9. KO Combined with Bif. animalis F1-7 Decreased the Gene Expression of Key Factors Regulating Inflammatory Response in the Intestine of Atherosclerosis Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Misra, B.B.; Puppala, S.R.; Comuzzie, A.G.; Mahaney, M.C.; VandeBerg, J.L.; Olivier, M.; Cox, L.A. Analysis of serum changes in response to a high fat high cholesterol diet challenge reveals metabolic biomarkers of atherosclerosis. PLoS ONE 2019, 14, e0214487. [Google Scholar] [CrossRef] [Green Version]
- Ben, J.; Jiang, B.; Wang, D.; Liu, Q.; Zhang, Y.; Qi, Y.; Tong, X.; Chen, L.; Liu, X.; Zhang, Y.; et al. Major vault protein suppresses obesity and atherosclerosis through inhibiting IKK-NF-κB signaling mediated inflammation. Nat. Commun. 2019, 10, 1801. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Sun, T.; Li, Y.; Zhang, D.; Zhou, J.; Su, X. Modulation of the Gut Microbiota by Krill Oil in Mice Fed a High-Sugar High-Fat Diet. Front. Microbiol. 2017, 8, 905. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Rosticci, M.; Morbini, M.; Cagnati, M.; Grandi, E.; Parini, A.; Borghi, C. Lipid-lowering and anti-inflammatory effects of omega 3 ethyl esters and krill oil: A randomized, cross-over, clinical trial. Archiv. Med. Sci. AMS 2016, 12, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food Res. 2017, 61, 1700098. [Google Scholar] [CrossRef]
- Ursoniu, S.; Sahebkar, A.; Serban, M.C.; Antal, D.; Mikhailidis, D.P.; Cicero, A.; Athyros, V.; Rizzo, M.; Rysz, J.; Banach, M. Lipid-modifying effects of krill oil in humans: Systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2017, 75, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Rundblad, A.; Holven, K.B.; Bruheim, I.; Myhrstad, M.C.; Ulven, S.M. Effects of krill oil and lean and fatty fish on cardiovascular risk markers: A randomised controlled trial. J. Nutr. Sci. 2018, 7, e3. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.; Lu, C.; Sun, T.; Zhou, J.; Li, Y.; Ming, T.; Bai, L.; Wang, Z.J.; Su, X. Alterations of the Brain Proteome and Gut Microbiota in d-Galactose-Induced Brain-Aging Mice with Krill Oil Supplementation. J. Agric. Food Chem. 2019, 67, 9820–9830. [Google Scholar] [CrossRef]
- O’Morain, V.L.; Ramji, D.P. The Potential of Probiotics in the Prevention and Treatment of Atherosclerosis. Mol. Nutr. Food Res. 2020, 64, e1900797. [Google Scholar] [CrossRef]
- Liang, X.; Lv, Y.Y.; Zhang, Z.; Yi, H.X.; Liu, T.J.; Li, R.; Yu, Z.; Zhang, L.W. Study on intestinal survival and cholesterol metabolism of probiotics. LWT—Food Sci. Technol. 2020, 124, 109132. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Wang, J.; Wu, F.; Sui, Y.; Yang, L.; Wang, Z. Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J. Dairy Sci. 2013, 96, 2746–2753. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhang, L.; Chen, H.; Feng, R.; Cao, P.; Liu, Y. Effects of Antarctic krill oil on lipid and glucose metabolism in C57BL/6J mice fed with high fat diet. Lipids Health Dis. 2017, 16, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.Y.; Ding, L.; Shi, H.H.; Xu, J.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Eicosapentaenoic acid in the form of phospholipids exerts superior anti-atherosclerosis effects to its triglyceride form in ApoE(-/-) mice. Food Funct. 2019, 10, 4177–4188. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-L.; Ou, H.-X.; Zhang, M.; Gong, D.; Zhao, Z.-W.; Chen, L.-Y.; Xia, X.-D.; Mo, Z.-C.; Tang, C.-K. Tanshinone IIA Promotes Macrophage Cholesterol Efflux and Attenuates Atherosclerosis of apoE-/- Mice by Omentin-1/ABCA1 Pathway. Curr. Pharm. Biotechnol. 2019, 20, 422–432. [Google Scholar] [CrossRef]
- Cremer, S.; Michalik, K.M.; Fischer, A.; Pfisterer, L.; Jaé, N.; Winter, C.; Boon, R.A.; Muhly-Reinholz, M.; John, D.; Uchida, S.; et al. Hematopoietic Deficiency of the Long Noncoding RNA MALAT1 Promotes Atherosclerosis and Plaque Inflammation. Circulation 2019, 139, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Hseu, Y.C.; Chang, Y.C.; Kumar, K.J.; Ho, T.Y.; Yang, H.L. Toona sinensis and its major bioactive compound gallic acid inhibit LPS-induced inflammation in nuclear factor-κB transgenic mice as evaluated by in vivo bioluminescence imaging. Food Chem. 2013, 136, 426–434. [Google Scholar] [CrossRef]
- Zhou, X.L.; Yan, B.B.; Xiao, Y.; Zhou, Y.M.; Liu, T.Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chem. Toxicol. 2018, 119, 296–301. [Google Scholar] [CrossRef]
- Stoehr, R.; Mavilio, M.; Marino, A.; Casagrande, V.; Kappel, B.; Moellmann, J.; Menghini, R.; Melino, G.; Federici, M. ITCH modulates SIRT6 and SREBP2 to influence lipid metabolism and atherosclerosis in ApoE null mice. Sci. Rep. 2015, 5, 9023. [Google Scholar] [CrossRef] [Green Version]
- Libby, G.R.G.P. Atherosclerosis and inflammation: Overview and updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar]
- Kitada, M.; Ogura, Y.; Koya, D. The protective role of Sirt1 in vascular tissue: Its relationship to vascular aging and atherosclerosis. Aging 2016, 8, 2290–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P.J.H. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids Health Dis. 2015, 14, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimstad, T.; Bjørndal, B.; Cacabelos, D.; Aasprong, O.G.; Janssen, E.A.; Omdal, R.; Svardal, A.; Hausken, T.; Bohov, P.; Portero-Otin, M.; et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scand. J. Gastroenterol. 2012, 47, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [Green Version]
- Sarkkinen, E.S.; Savolainen, M.J.; Taurio, J.; Marvola, T.; Bruheim, I. Prospective, randomized, double-blinded, placebo-controlled study on safety and tolerability of the krill powder product in overweight subjects with moderately elevated blood pressure. Lipids Health Dis. 2018, 17, 287. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Smith, A.D.; Solano-Aguilar, G.; Wang, T.T.Y.; Pham, Q.; Beshah, E.; Tang, Q.; Urban, J.F.; Xue, C.; Li, R.W. Mechanistic insights into the attenuation of intestinal inflammation and modulation of the gut microbiome by krill oil using in vitro and in vivo models. Microbiome 2020, 8, 1–21. [Google Scholar] [CrossRef]
- Kanmani, P.; Kim, H. Functional capabilities of probiotic strains on attenuation of intestinal epithelial cell inflammatory response induced by TLR4 stimuli. Biofactors 2019, 45, 223–235. [Google Scholar] [CrossRef]
- Din, A.U.; Hassan, A.; Zhu, Y.; Zhang, K.; Wang, Y.; Li, T.; Wang, Y.; Wang, G. Inhibitory effect of Bifidobacterium bifidum ATCC 29521 on colitis and its mechanism. J. Nutr. Biochem. 2020, 79, 108353. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, S.S.; Lee, M.; Lee, E.H.; Rhee, I.; Chang, S.Y.; Lim, S.J. Krill Oil-Incorporated Liposomes As An Effective Nanovehicle To Ameliorate The Inflammatory Responses Of DSS-Induced Colitis. Int. J. Nanomed. 2019, 14, 8305–8320. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.S.; Ju, J.H.; Lee, J.E.; Park, H.J.; Lee, J.M.; Shin, H.K.; Holzapfel, W.; Park, K.Y.; Do, M.S. The probiotic Lactobacillus rhamnosus BFE5264 and Lactobacillus plantarum NR74 promote cholesterol efflux and suppress inflammation in THP-1 cells. J. Sci. Food Agric. 2013, 93, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Renuka; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front. Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, H.; Zhao, Z.J.; Xiao, S.; Zhao, Y.J.; Duan, C.C.; Gao, L.; Li, S.Y.; Wang, J.H. Pediococcus acidilactici AS185 attenuates early atherosclerosis development through inhibition of lipid regulation and inflammation in rats. J. Funct. Foods 2019, 60, 103424. [Google Scholar] [CrossRef]
- Judit, C.; Laura, P.-P.; Lorena, C.-P.; Elisabet, L.; Rosa, S.; Anna, P.; Valls, R.M. Fermented Dairy Products, Probiotic Supplementation, and Cardiometabolic Diseases: A Systematic Review and Meta-analysis. Adv. Nutr. 2020, 4, 834–863. [Google Scholar]
- Sayin, S.I.; Wahlstrom, A.; Felin, J.; Jantti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyotylainen, T.; Oresic, M.; Backhed, F. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-beta-muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Yang, X.; Long, Y.; Zhong, H.; Wang, P.; Yuan, P.; Zhang, X.; Che, L.; Feng, B.; Li, J.; et al. Dietary supplementation withLactobacillus plantarummodified gut microbiota, bile acid profile and glucose homoeostasis in weaning piglets. Br. J. Nutr. 2020, 124, 797–808. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Z.; Zhou, X.; Lu, Y.; Li, R.; Yu, Z.; Tong, L.; Gong, P.; Yi, H.; Liu, T.; et al. Probiotics improved hyperlipidemia in mice induced by a high cholesterol diet via downregulating FXR. Food Funct. 2020, 11, 9903–9911. [Google Scholar] [CrossRef]
- Tandy, S.; Chung, R.W.S.; Wat, E.; Kamili, A.; Berge, K.; Griinari, M.; Cohn, J.S. Dietary Krill Oil Supplementation Reduces Hepatic Steatosis, Glycemia, and Hypercholesterolemia in High-Fat-Fed Mice. J. Agric. Food Chem. 2009, 57, 9339–9345. [Google Scholar] [CrossRef]
- Cui, C.; Li, Y.; Gao, H.; Zhang, H.; Han, J.; Zhang, D.; Li, Y.; Zhou, J.; Lu, C.; Su, X. Modulation of the gut microbiota by the mixture of fish oil and krill oil in high-fat diet-induced obesity mice. PLoS ONE 2017, 12, e0186216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liang, X.; Lv, Y.Y.; Yi, H.X.; Chen, Y.J.; Bai, L.; Zhou, H.; Liu, T.J.; Li, R.; Zhang, L.W. Evaluation of probiotics for improving and regulation metabolism relevant to type 2 diabetes in vitro. J. Funct. Foods 2020, 64, 103664. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhang, Z.; Tong, L.J.; Zhou, X.H.; Liang, X.; Yi, H.X.; Gong, P.M.; Liu, T.J.; Zhang, L.W.; Yang, L.Q.; et al. Mechanisms underlying the promotion of 5-hydroxytryptamine secretion in enterochromaffin cells of constipation mice by Bifidobacterium and Lactobacillus. Neurogastroenterol. Motil. 2021, 33, e14082. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Singh, B.P.; Saini, K.; Kumari, M.; Tomar, S.K.; Mishra, V. Role of microbes, metabolites and effector compounds in host-microbiota interaction: A pharmacological outlook. Environ. Chem. Lett. 2019, 17, 1801–1820. [Google Scholar] [CrossRef]









| Normal Diet | High Fat Diet | |
|---|---|---|
| Nutrient and energy composition | Caloric (kcal/100 g) 352 | Caloric (kcal/100 g) 524 |
| Carbohydrates (g/100 g) 52 | Carbohydrates (g/100 g) 20 | |
| Energy (%) 63.9 | Energy (%) 20 | |
| Proteins (g/100 g) 20.1 | Proteins (g/100 g) 28 | |
| Energy (%) 20.3 | Energy (%) 35 | |
| Lipids (g/100 g) 5.9 | Lipids (g/100 g) 33 | |
| Energy (%) 15.8 | Energy (%) 45 | |
| (g/kg) | Casein 200 | Casein 200 |
| l-Cystine 3 | l-Cystine 3 | |
| Corn starch 397 | Corn starch 72.8 | |
| Maltodextrin 132 | Maltodextrin 100 | |
| Sucrose 100 | Sucrose 172.8 | |
| Cellulose 50 | Cellulose 50 | |
| Soybean Oil 70 | Soybean Oil 25 | |
| t-Butylhydroquinone 0.014 | t-Butylhydroquinone 25 | |
| Lard Oil 0 | Lard Oil 177.50 | |
| Mineral Mix 35 | Mineral Mix 10 | |
| Vitamin Mix 10 | Vitamin Mix 10 | |
| Choline Bitartrate 2.5 | Choline Bitartrate 2 | |
| Potassium Bitartrate 0 | Potassium Bitartrate 16.50 | |
| Calcium Carbonate 0 | Calcium Carbonate 5.5 | |
| FD&C Red Dye 0 | FD&C Red Dye 0.05 | |
| Di Calcium Phosphate 0 | Di Calcium Phosphate 13 |
| Gene Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | ACTGCTCTGGCTCCTAGCAC | CCACCGATCCACACAGAGTA |
| FXR | ACAGAGAGGCGGTGGAGAAGC | TCAGCGTGGTGATGGTTGAATGTC |
| FGF15 | CCTGTACTCCGCTGGTCCCTATG | GGTCCTCCTCGCAGTCCACAG |
| TLR4 | CACAGAAGAGGCAAGGCGACAG | GAATGACCCTGACTGGCACTAACC |
| MYD88 | AGCAGAACCAGGAGTCCGAGAAG | GGGCAGTAGCAGATAAAGGCATCG |
| NF-κB | TCGAGTCTCCATGCAGCTACGG | CGGTGGCGATCATCTGTGTCTG |
| TNF-α | GGACTAGCCAGGAGGGAGAACAG | GCCAGTGAGTGAAAGGGACAGAAC |
| IL-1β | TCGCAGCAGCACATCAACAAGAG | AGGTCCACGGGAAAGACACAGG |
| Occludin | ACCCGAAGAAAGATGGATCG | CATAGTCAGATGGGGGTGGA |
| ZO-1 | CTTCTCTTGCTGGCCCTAAAC | TGGCTTCACTTGAGGTTTCTG |
| Muc-2 | ATGCCCACCTCCTCAAAGAC | GTAGTTTCCGTTGGAACAGTGAA |
| CYP7A1 | GTGTAGAGGCTGGAGGTGATGTTG | AAGGGCACTGCGGCAAGTTG |
| Compositions and Properties | % | |
|---|---|---|
| Fatty acid | C12:0 | 0.13 |
| C14:0 | 9.06 | |
| C16:0 | 12.32 | |
| C16:1 | 5.96 | |
| C17:0 | 0.11 | |
| C18:0 | 1.63 | |
| C18:1 | 10.91 | |
| C18:2 | 1.92 | |
| C18:3 | 1.52 | |
| C22:0 | 0.09 | |
| C20:4 | 1.01 | |
| EPA C20:5 | 29.36 | |
| DHA C22:6 | 19.02 | |
| Phospholipids (%) | 63.54 | |
| Astaxanthin(mg/100 g) | 190.25 | |
| Acid value (mg KOH/g) | 6.23 | |
| Peroxide value (meq/kg) | 2.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Zhang, Z.; Lv, Y.; Lu, H.; Liu, T.; Yi, H.; Zhao, M.; Zhang, L.; Gong, P. Krill Oil Combined with Bifidobacterium animalis subsp. lactis F1-7 Alleviates the Atherosclerosis of ApoE−/− Mice. Foods 2021, 10, 2374. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102374
Liang X, Zhang Z, Lv Y, Lu H, Liu T, Yi H, Zhao M, Zhang L, Gong P. Krill Oil Combined with Bifidobacterium animalis subsp. lactis F1-7 Alleviates the Atherosclerosis of ApoE−/− Mice. Foods. 2021; 10(10):2374. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102374
Chicago/Turabian StyleLiang, Xi, Zhe Zhang, Youyou Lv, Haiyan Lu, Tongjie Liu, Huaxi Yi, Maozhen Zhao, Lanwei Zhang, and Pimin Gong. 2021. "Krill Oil Combined with Bifidobacterium animalis subsp. lactis F1-7 Alleviates the Atherosclerosis of ApoE−/− Mice" Foods 10, no. 10: 2374. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102374