Traditional Fermented Beverages of Mexico: A Biocultural Unseen Foodscape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Map Construction
2.3. Network Analysis
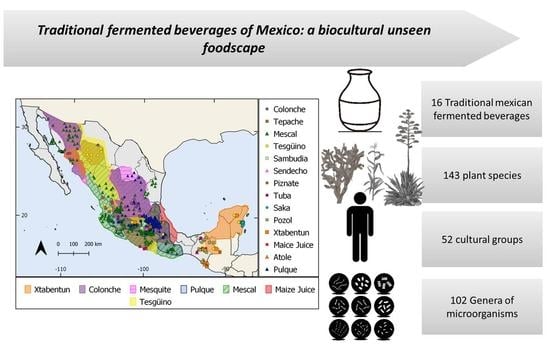
3. Results
3.1. Traditional Mexican Fermented Beverages
3.2. Current State of the Conceptual Overview
3.3. Plant Diversity Used in Mexican Traditional Fermented Beverages
3.3.1. Cluster of Maize Beverages
3.3.2. Cluster of Agave Beverages
3.3.3. Cluster of Cacti Beverages
3.3.4. Cluster of Palm Beverages
3.3.5. Balché Cluster Beverages
3.3.6. The Cluster of Tepache and Sambudia
3.4. Traditional Fermented Beverages as Dynamic Systems: The Addition of New Substrates
3.5. Traditional Knowledge and Microbial Communities in Fermented Beverages: How Do Traditional Fermenters Promote Microbial Reservoirs and Microbial Diversity
How Diverse Are the Inconspicuous Microbial Environments?
3.6. Uses of Fermented Beverages by Human Cultural Groups and Future Directions
3.7. Reviewed Distribution of the TMFB
3.8. How Are We Attending the Maintenance of This Intangible Biocultural Heritage?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blandino, A.; Al-Aseeri, M.E.; Pandiella, S.S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Dudley, R. The Fruits of Fermentation. In The Drunken Monkey; University of California Press: Berkley, CA, USA, 2014; pp. 11–33. [Google Scholar]
- Gibbons, J.G.; Rinker, D.C. The genomics of microbial domestication in the fermented food environment. Curr. Opin. Genet. Dev. 2015, 35, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lévi-Strauss, C. The Culinary Triangle. In Levi-Strauss: Structuralism and Dialectics; Paidos: Buenos Aires, Argentina, 1968; pp. 39–57. [Google Scholar]
- Achi, O.K. The potential for upgrading traditional fermented foods through biotechnology. Afr. J. Biotechnol. 2005, 4, 375–380. [Google Scholar]
- Marsh, A.J.; Hill, C.; Ross, R.P.; Cotter, P.D. Fermented beverages with health-promoting potential: Past and future perspectives. Trends Food Sci. Technol. 2014, 38, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Nabhan, G.P. Ethnobiology for a Diverse World: Microbial Ethnobiology and the Loss of Distinctive Food Cultures. J. Ethnobiol. 2010, 30, 181–183. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Nisbett, N.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The future of the global food system. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2769–2777. [Google Scholar] [CrossRef] [Green Version]
- Battcock, M. Fermented Fruits and Vegetables: A Global Perspective; No. 134; FAO: Rome, Italy, 1998. [Google Scholar]
- Pieroni, A.; Vandebroek, I. Traveling Cultures and Plants: The Ethnobiology and Ethnopharmacy of Migrations; Berghahn Books: New York, NY, USA, 2007; Volume 7. [Google Scholar]
- Steinkraus, K.H. Nutritional significance of fermented foods. Food Res. Int. 1994, 27, 259–267. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2019, 19, 184–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinkraus, K.H. Classification of fermented foods: Worldwide review of household fermentation techniques. Food Control 1997, 8, 311–317. [Google Scholar] [CrossRef]
- Tamang, J.; Samuel, D. Dietary Cultures and Antiquity of Fermented Foods and Beverages. In Fermented Foods and Beverages of the World; Tamang, J.P., Kailasapathy, K., Eds.; CRC Press: New York, NY, USA, 2010; pp. 1–40. [Google Scholar]
- Legras, J.L.; Merdinoglu, D.; Cornuet, J.M.; Karst, F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Mol. Ecol. 2007, 16, 2091–2102. [Google Scholar] [CrossRef]
- Valamoti, S.M. Brewing beer in wine country? First archaeobotanical indications for beer making in Early and Middle Bronze Age Greece. Veg. Hist. Archaeobot. 2018, 27, 611–625. [Google Scholar] [CrossRef]
- Campbell-Platt, G. Fermented foods—A world perspective. Food Res. Int. 1994, 27, 253–257. [Google Scholar] [CrossRef]
- Hassan, A.N.; Frank, J.F. Starter cultures and their use. In Applied Dairy Microbiology; CRC Press: Plymouth, UK, 2001; pp. 171–226. [Google Scholar]
- Zorba, M.; Hancioglu, O.; Genc, M.; Karapinar, M.; Ova, G. The use of starter cultures in the fermentation of boza, a traditional Turkish beverage. Process. Biochem. 2003, 38, 1405–1411. [Google Scholar] [CrossRef]
- Kebede, A.; Viljoen, B.; Gadaga, T.; Narvhus, J.; Lourens-Hattingh, A. The effect of container type on the growth of yeast and lactic acid bacteria during production of Sethemi, South African spontaneously fermented milk. Food Res. Int. 2007, 40, 33–38. [Google Scholar] [CrossRef]
- Väkeväinen, K.; Hernández, J.; Simontaival, A.-I.; Severiano-Pérez, P.; Díaz-Ruiz, G.; von Wright, A.; Wacher-Rodarte, C.; Plumed-Ferrer, C. Effect of different starter cultures on the sensory properties and microbiological quality of Atole agrio, a fermented maize product. Food Control 2019, 109, 106907. [Google Scholar] [CrossRef]
- Ojeda-Linares, C.I.; Vallejo, M.; Lappe-Oliveras, P.; Casas, A. Traditional management of microorganisms in fermented beverages from cactus fruits in Mexico: An ethnobiological approach. J. Ethnobiol. Ethnomed. 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Casas, A. Physical, Chemical, and Microbiological Characteristics of Pulque: Management of a Fermented Beverage in Michoacán, Mexico. Foods 2020, 9, 361. [Google Scholar] [CrossRef] [Green Version]
- Gadaga, T.H.; Mutukumira, A.N.; Narvhus, J.A.; Feresu, S.B. A review of traditional fermented foods and beverages of Zimbabwe. Int. J. Food Microbiol. 1999, 53, 1–11. [Google Scholar] [CrossRef]
- Tamang, J. Diversity of Fermented Beverages and Alcoholic Drinks. In Fermented Foods and Beverages of the World; Routledge: New York, NY, USA, 2010; pp. 85–125. [Google Scholar] [CrossRef]
- Tamang, J.P.; Thapa, S.; Tamang, N.; Rai, B. Indigenous fermented food beverages of Darjeeling hills and Sikkim: A process and product characterization. J. Hill Res. 1996, 9, 401–411. [Google Scholar]
- Tamang, J.P.; Tamang, N.; Thapa, S.; Dewan, S.; Tamang, B.; Yonzan, H.; Kharel, N. Microorganisms and nutritional value of ethnic fermented foods and alcoholic beverages of Northeast India. Indian J. Tradit. Knowl. 2012, 11, 7–25. [Google Scholar]
- Madej, T.; Pirożnikow, E.; Dumanowski, J.; Łuczaj, Ł. Juniper beer in Poland: The story of the revival of a traditional bever-age. J. Ethnobiol. 2014, 34, 84–103. [Google Scholar] [CrossRef]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.-L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.X.; Aguilar, A. Sustainable diets: Social and cultural perspectives. In Sustainable Diets: Linking Nutrition and Food Systems; CABI: Wallingford, UK, 2019; p. 131. [Google Scholar]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Smýkal, P.; Nelson, M.N.; Berger, J.D.; Von Wettberg, E.J. The impact of genetic changes during crop domestication. Agronomy 2018, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Gaut, B.S.; Seymour, D.K.; Liu, Q.; Zhou, Y. Demography and its effects on genomic variation in crop domestication. Nat. Plants 2018, 4, 512–520. [Google Scholar] [CrossRef]
- Von Wettberg, E.; Davis, T.M.; Smýkal, P. Wild Plants as Source of New Crops. Front. Plant Sci. 2020, 11, 1426. [Google Scholar] [CrossRef]
- Blancas, J.; Casas, A.; Moreno-Calles, A.I.; Caballero, J. Cultural motives of plant management and domestication. In Ethnobotany of Mexico; Springer: New York, NY, USA, 2016; pp. 233–255. [Google Scholar]
- Caballero, J.; Casas, A.; Cortés, L.; Mapes, C. Patrones en el conocimiento, uso y manejo de plantas en pueblos indígenas de México. Estud. Atacameños 1998, 16, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Casas, A.; Torres-Guevara, J.; Parra, F. Domesticación y en el Continente Americano. Manejo de Biodiveridad y Evolución Dirigida por las Culturas del Nuevo Mundo; Universidad Nacional Autónoma de México; Universidad Nacional Agraria La Molina: Mexico City, Mexico, 2016; Volume 1, pp. 25–50. [Google Scholar]
- Casas, A.; Parra, F.; Rangel-Landa, S.; Blancas, J.; Vallejo, M.; Moreno-Calles, A.I.; Guillén, S.; Torres-García, I.; Delgado-Lemus, A.; Pérez-Negrón, E.; et al. Manejo y Domesticación de Plantas en Mesoamérica. Una Estrategia de Investigación y Estado del Conocimiento Sobre los Recursos Genéticos de México; Universidad Nacional Autónoma de México; Universidad Nacional Agraria La Molina: Mexico City, Mexico, 2016; Volume 1. [Google Scholar]
- Linares, M.E.; Bye, R. Las especies subutilizadas de la milpa. Rev. Digit. Univ. 2015, 16, 22. [Google Scholar]
- Scovazzi, T. The Definition of Intangible Cultural Heritage. In Cultural Heritage, Cultural Rights, Cultural Diversity; Brill Nijhoff: Leiden, The Netherlands, 2012; pp. 179–200. [Google Scholar]
- Romaine, S.; Gorenflo, L.J. Linguistic diversity of natural UNESCO world heritage sites: Bridging the gap between nature and culture. Biodivers. Conserv. 2017, 26, 1973–1988. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.; McKevitt, A. Laws and Regulations of Traditional Foods: Past, Present and Future. In Traditional Foods; Springer: Cham, Switzerland, 2019; pp. 239–271. [Google Scholar]
- Kühne, B.; Vanhonacker, F.; Gellynk, X.; Verbeke, W. Innovation in traditional food products in Europe: Do sector innovation activities match consumers’ acceptance? Food Qual. Prefer. 2010, 21, 629–638. [Google Scholar] [CrossRef]
- Gellynck, X.; Kühne, B. Innovation and collaboration in traditional food chain networks. J. Chain Netw. Sci. 2008, 8, 121–129. [Google Scholar] [CrossRef]
- Guerrero, L.; Guàrdia, M.D.; Xicola, J.; Verbeke, W.; Vanhonacker, F.; Zakowska-Biemans, S.; Sajdakowska, M.; Sulmont-Rossé, C.; Issanchou, S.; Contel, M.; et al. Consumer-driven definition of traditional food products and innovation in traditional foods. A qualitative cross-cultural study. Appetite 2009, 52, 345–354. [Google Scholar] [CrossRef]
- Romero-Luna, H.E.; Hernández-Sánchez, H.; Dávila-Ortiz, G. Traditional fermented beverages from Mexico as a potential probiotic source. Ann. Microbiol. 2017, 67, 577–586. [Google Scholar] [CrossRef]
- Ramírez-Guzmán, K.N.; Torres-León, C.; Martinez-Medina, G.A.; de la Rosa, O.; Hernández-Almanza, A.; Alvarez-Perez, O.B.; Araujo, R.; González, L.R.; Londoño, L.; Ventura, J.; et al. Traditional Fermented Beverages in Mexico. In Fermented Beverages; Woodhead Publishing: Sawston, UK, 2019; pp. 605–635. [Google Scholar]
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef]
- Robledo-Márquez, K.; Ramírez, V.; González-Córdova, A.; Ramírez-Rodríguez, Y.; García-Ortega, L.; Trujillo, J. Research opportunities: Traditional fermented beverages in Mexico. Cultural, microbiological, chemical, and functional aspects. Food Res. Int. 2021, 147, 110482. [Google Scholar] [CrossRef]
- Godoy, A.; Herrera, T.; Ulloa, M. Más allá del Pulque y el Tepache: Las Bebidas Alcohólicas no Destiladas Indígenas de México; Unam: Mexico City, Mexico, 2003; Volume 1. [Google Scholar]
- Wilson, I.; Pineda, A. Pineda’s Report on the Beverages of New Spain. Arizona West 1963, 5, 79. Available online: http://0-www-jstor-org.brum.beds.ac.uk/stable/40167046 (accessed on 18 August 2021).
- Bruman, H.J. Alcohol in Ancient Mexico; University of Utah Press: Salt Lake City, UT, USA, 2000. [Google Scholar]
- Enríquez-Salazar, M.I.; Veana, F.; Aguilar, C.N.; De la Garza-Rodríguez, I.M.; López, M.G.; Rutiaga-Quiñones, O.M.; Morlett-Chávez, J.A.; Rodríguez-Herrera, R. Microbial diversity and biochemical profile of aguamiel collected from Agave salmiana and A. atrovirens during different seasons of year. Food Sci. Biotechnol. 2017, 26, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Ben Omar, N.; Ampe, F. Microbial Community Dynamics during Production of the Mexican Fermented Maize Dough Pozol. Appl. Environ. Microbiol. 2000, 66, 3664–3673. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Arriaga, C.; Espinal-Centeno, A.; Martinez-Sánchez, S.; Caballero-Pérez, J.; Alcaraz, L.D.; Cruz-Ramírez, A. Deep microbial community profiling along the fermentation process of pulque, a biocultural resource of Mexico. Microbiol. Res. 2020, 241, 126593. [Google Scholar] [CrossRef]
- Chacón-Vargas, K.; Torres, J.; Giles-Gómez, M.; Escalante, A.; Gibbons, J.G. Genomic profiling of bacterial and fungal communities and their predictive functionality during pulque fermentation by whole-genome shotgun sequencing. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Elizaquível, P.; Carrasco, P.; Espinosa, J.; Reyes, D.; Wacher, C.; Aznar, R. Diversity and dynamics of lactic acid bacteria in Atole agrio, a traditional maize-based fermented beverage from South-Eastern Mexico, analysed by high throughput sequencing and culturing. Antonie Van Leeuwenhoek 2018, 111, 385–399. [Google Scholar] [CrossRef]
- Astudillo-Melgar, F.; Ochoa-Leyva, A.; Utrilla, J.; Huerta-Beristain, G. Bacterial Diversity and Population Dynamics During the Fermentation of Palm Wine from Guerrero Mexico. Front. Microbiol. 2019, 10, 531. [Google Scholar] [CrossRef]
- Villarreal-Morales, S.L.; Montañez-Saenz, J.C.; Aguilar-González, C.N.; Rodriguez-Herrera, R. Metagenomics of traditional beverages. In Advances in Biotechnology for Food Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 301–326. [Google Scholar]
- Toledo, V.M.; Alarcón-Chaires, P.; Moguel, P.; Olivo, M.; Cabrera, A.; Leyequien, E.; Rodríguez-Aldabe, A. El atlas etnoeco-lógico de México y Centroamérica: Fundamentos, métodos y resultados. Etnoecológica 2001, 6, 7–41. [Google Scholar]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2009, 84, 523–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedi, Z.; Van Eck, N.J. Visualizing readership activity of Mendeley users using VOSviewer. In Proceedings of the Altmetrics14: Expanding Impacts and Metrics, Workshop at Conference, Bloomington, IN, USA, 24–26 June 2014; Volume 1041819. [Google Scholar]
- Blomberg, Á.; de Norma, A.; Moreno, D. Distribución de las Lenguas Indígenas de México; Jardín Etnobotánico de Oaxaca—Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2008; Volume 1. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 8 March 2021).
- R Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2020; Available online: http://www.rstudio.com (accessed on 17 March 2021).
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Nuraida, L.; Wacher, M.C.; Owens, J.D. Microbiology of pozol, a Mexican maize dough. World J. Microbiol. Biotechnol. 1995, 11, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Sanchez, C.; O’sullivan, D.J.; McKay, L.L. Classification of a bacterial isolate, from pozol, exhibiting antimicrobial activity against several gram-positive and gram-negative bacteria, yeasts, and molds. J. Food Prot. 2000, 63(8), 1123–1132. [Google Scholar] [CrossRef]
- Larroque, M.; Carrau, F.; Fariña, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef]
- Wacher, C.; Cañas, A.; Bárzana, E.; Lappe, P.; Ulloa, M.; Owens, J.D. Microbiology of Indian and Mestizo pozol fermentations. Food Microbiol. 2000, 17, 251–256. [Google Scholar] [CrossRef]
- De la Luz, S. Memoria Alimentaria y transición cultural en la comunidad Mazahua de San Pedro de los baños, Ixtlahuaca, Estado de México. Bachelor’s Thesis, Universidad Autónoma del Estado de México, Ciudad de México, Mexico, 2019. [Google Scholar]
- Jiménez Vera, R.; Gonzalez Cortes, N.; Magaña Contreras, A.; Corona Cruz, A. Microbiological and sensory evaluation of fermented white pozol, with cacao (Theobroma cacao) and coconut (Cocos nucifera). Rev. Venez. Cienc. Tecnol. Aliment. 2010, 1, 70–80. [Google Scholar]
- Velázquez-López, A.; Covatzin-Jirón, D.; Toledo-Meza, M.D.; Vela-Gutiérrez, G. Fermented drink elaborated with lactic acid bacteria isolated from chiapaneco traditional pozol. Cienc. UAT 2018, 13, 165–178. [Google Scholar]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdes, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. LWT 2019, 112, 108236. [Google Scholar] [CrossRef]
- Trabanino, F.; Meléndez, L. El ajkum sa’o’pozol de camote’-una bebida entre los mayas palencanos del Clásico Tardío. Ketzalcalli 2016, 2, 3–21. [Google Scholar]
- Wacher, C.; Canas, A.; Cook, P.E.; Bárzana, E.; Owens, J.D. Sources of microorganisms in pozol, a traditional Mexican fer-mented maize dough. World J. Microbiol. Biotechnol. 1993, 9, 269–274. [Google Scholar] [CrossRef]
- Díaz-Ruiz, G.; Guyot, J.; Ruiz-Teran, F.; Morlon-Guyot, J.; Wacher, C. Microbial and physiological characterization of weak-ly amylolytic but fast-growing lactic acid bacteria: A functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl. Environ. Microbiol. 2003, 69, 4367–4374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar-Ramírez, M.C.; Jaimez-Ordaz, J.; Escorza-Iglesias, V.A.; Rodríguez-Serrano, G.M.; Contreras-López, E.; Ramírez-Godínez, J.; González-Olivares, L.G. Lactobacillus pentosus ABHEAU-05: An in vitro digestion resistant lactic acid bacte-rium isolated from a traditional fermented Mexican beverage. Rev. Argent. Microbiol. 2020, 52, 305–314. [Google Scholar] [CrossRef]
- Guadarrama Orozco, K.D. Tipificación de Bacterias Lácticas Aisladas del Axocotl, Atole Agrio de la Sierra Norte de Puebla, por Medio de ADRA. Bachelor’s Thesis, Facutlad de Química Universidad Nacional Autónoma de México, Mexico City, Mexico, 2007. [Google Scholar]
- Ampe, F.; ben Omar, N.; Moizan, C.; Wacher, C.; Guyot, J.-P. Polyphasic Study of the Spatial Distribution of Microorganisms in Mexican Pozol, a Fermented Maize Dough, Demonstrates the Need for Cultivation-Independent Methods To Investigate Traditional Fermentations. Appl. Environ. Microbiol. 1999, 65, 5464–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar Colmenares, S. Reivindicación de la dieta de la milpa y otros alimentos que la complementan en la región los Altos de Chiapas; Universidad de Ciencias y Artes de Chiapas: Tuxtla Gutiérrez, Chiapas, Mexico, 2019. [Google Scholar]
- Silva, L.M.L.; Trabanino, F. Preparación de pozol de camote con maíz en Palenque, Chiapas. Antrópica Rev. Cienc. Soc. Humanid. 2018, 4, 159–169. [Google Scholar]
- McGee, R.J. The balché ritual of the Lacandon Maya. Estud. Cult. Maya 2013, 18, 439–457. [Google Scholar]
- Lappe, P.; Ulloa, M.; Gómez, J. Microbial and chromatographic study of tejuino from Jalisco, Mexico. Rev. Mex. Microbiol. 1989, 5, 181–203. [Google Scholar]
- Valdez, A.V.; Garcia, L.E.S.; Kirchmayr, M.; Rodríguez, P.R.; Esquinca, A.G.; Coria, R.; Mathis, A.G. Yeast communities associated with artisanal mezcal fermentations from Agave salmiana. Antonie Van Leeuwenhoek 2011, 100, 497–506. [Google Scholar] [CrossRef]
- Lappe, P.; Ulloa, M. Estudios Étnicos, Microbianos y Químicos del Tesgüino Tarahumara; UNAM: Mexico City, Mexico, 1989; Volume 1. [Google Scholar]
- Cruz, S.; Ulloa, M. Alimentos Fermentados de Maíz Consumidos en México y Otros Países Latinoamericanos; UNAM: Mexico City, Mexico, 1989; Volume 1. [Google Scholar]
- Castro, J.T. Bebidas fermentadas indígenas: Cacao, pozol, tepaches, tesgüino y tejuino. In Conquista y Comida: Consecuencias del Encuentro de dos Mundos; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1997; pp. 437–448. [Google Scholar]
- Moreno-Fuentes, Á.; Aguirre-Acosta, E.; Pérez-Ramírez, L. Conocimiento tradicional y científico de los hongos en el estado de Chihuahua, México. Etnobiología 2004, 4, 89–117. [Google Scholar]
- Väkeväinen, K.; Valderrama, A.; Espinosa, J.; Centurión, D.; Rizo, J.; Reyes-Duarte, D.; Plumed-Ferrer, C. Characterization of lactic acid bacteria recovered from atole agrio, a traditional Mexican fermented beverage. LWT 2018, 88, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez Rojas, D. Por el Camino Real de la Costa: Apuntes sobre la tradición mariachera en la costa de Michoacán. Relac. Estud. Hist. Soc. 2014, 35(139), 281–304. [Google Scholar] [CrossRef] [Green Version]
- Escalante, A.; López Soto, D.R.; Velázquez Gutiérrez, J.E.; Giles-Gómez, M.; Bolívar, F.; López-Munguía, A. Pulque, a tradi-tional Mexican alcoholic fermented beverage: Historical, microbiological, and technical aspects. Front. Microbiol. 2016, 7, 1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Rivas, E.; Rendón-Domínguez, A.; Felipe-Salinas, J.A.; Cuffia, F. What is gastronomy? An exploratory study of social representation of gastronomy and Mexican cuisine among experts and consumers using a qualitative approach. Food Qual. Preference 2020, 83, 103930. [Google Scholar] [CrossRef]
- Yañez, S.B.; Ortíz, H.T.; Ortega, A.E.; Bordi, I.V. Venta informal de pulque como estrategia de reproducción social. Evidencias del centro de México. Rev. Geogr. Agrícola 2019, 62, 49–67. [Google Scholar]
- Chandrasekhar, K.; Sreevani, S.; Seshapani, P.; Pramodhakumari, J. A review on palm wine. Int. J. Res. Biol. Sci. 2012, 2, 33–38. [Google Scholar]
- Ruiz-Terán, F.; Martínez-Zepeda, P.N.; Geyer-de la Merced, S.Y.; Nolasco-Cancino, H.; Santiago-Urbina, J.A. Mezcal: In-digenous Saccharomyces cerevisiae strains and their potential as starter cultures. Food Sci. Biotechnol. 2019, 28, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Urbina, J.A.; Ruíz-Terán, F. Microbiology and biochemistry of traditional palm wine produced around the world. Int. Food Res. J. 2014, 21, 1261–1269. [Google Scholar]
- Santiago-Urbina, J.A.; Arias-García, J.A.; Ruiz-Terán, F. Yeast species associated with spontaneous fermentation of taberna, a traditional palm wine from the southeast of Mexico. Ann. Microbiol. 2015, 65, 287–296. [Google Scholar] [CrossRef]
- Sánchez, D.G.; Ríos, G.L. Manejo de la palma de coco (Cocos nucifera L.) en México. Revista Chapingo. Serie ciencias forestales y del ambiente. Rev. Chapingo Ser. Cienc. For. Ambiente 2002, 8(1), 39. [Google Scholar]
- De Albuquerque, U.P. Re-examining hypotheses concerning the use and knowledge of medicinal plants: A study in the Caatinga vegetation of NE Brazil. J. Ethnobiol. Ethnomed. 2006, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandebroek, I.; Balick, M.J. Globalization and loss of plant knowledge: Challenging the paradigm. PLoS ONE 2012, 7(5), e37643. [Google Scholar] [CrossRef] [PubMed]
- Ceuterick, M.; Vandebroek, I.; Torry, B.; Pieroni, A. Cross-cultural adaptation in urban ethnobotany: The Colombian folk pharmacopoeia in London. J. Ethnopharmacol. 2008, 120, 342–359. [Google Scholar] [CrossRef]
- Ceuterick, M.; Vandebroek, I.; Pieroni, A. Resilience of Andean urban ethnobotanies: A comparison of medicinal plant use among Bolivian and Peruvian migrants in the United Kingdom and in their countries of origin. J. Ethnopharmacol. 2011, 136, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Van Andel, T.; Westers, P. Why Surinamese migrants in the Netherlands continue to use medicinal herbs from their home country. J. Ethnopharmacol. 2010, 127, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Moore, P.H.; Tew, T.L. The Gene Pool of Saccharum Species and Their Improvement. In Genomics of the Saccharinae; Springer: New York, NY, USA, 2013; pp. 43–71. [Google Scholar]
- Bennett, B.; Prance, G.T. Introduced plants in the indigenous Pharmacopoeia of Northern South America. Econ. Bot. 2000, 54, 90–102. [Google Scholar] [CrossRef]
- Kumarathilake, D.M.H. Study on the Extinction Risks, Conservation and Domestication of Endemic Wild Cinnamomum Species in Sri Lanka. Master’s Thesis, University of Ruhuna, Ruhuna, Sri Lanka, 2012. [Google Scholar]
- Ranasinghe, P.; Pigera, S.; Premakumara, G.S.; Galappaththy, P.; Constantine, G.R.; Katulanda, P. Medicinal properties of ‘true’cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complem. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titilayo, F.; Temitope, A. Microbiological and Physicochemical Changes in Palm Wine Subjected to Spontaneous Fermentation During Storage. Int. J. Biotechnol. 2019, 8, 48–58. [Google Scholar]
- Djeni, T.N.; Kouame, K.H.; Ake, F.D.M.; Amoikon, L.S.T.; Dje, M.K.; Jeyaram, K. Microbial Diversity and Metabolite Profiles of Palm Wine Produced from Three Different Palm Tree Species in Côte d’Ivoire. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Zizumbo-Villarreal, D. History of coconut (Cocos nucifera L.) in Mexico: 1539? Genet. Resour. Crop. Evol. 1996, 43, 505–515. [Google Scholar] [CrossRef]
- Zizumbo-Villarreal, D.; Colunga-Garcíamarín, P. Early coconut distillation and the origins of mezcal and tequila spirits in west-central Mexico. Genet. Resour. Crop. Evol. 2007, 55, 493–510. [Google Scholar] [CrossRef]
- Zizumbo-Villarreal, D.; González-Zozaya, F.; Olay-Barrientos, A.; Almendros-López, L.; Flores-Pérez, P.; Colunga-Garcíamarín, P. Distillation in Western Mesoamerica before European Contact. Econ. Bot. 2009, 63, 413–426. [Google Scholar] [CrossRef]
- Machuca, P. El Vino de Cocos en la Nueva España. Historia de una Transculturación en el Siglo XVII; Colegio de Michoacán: Zamora, Mexico, 2018. [Google Scholar]
- Corona-González, R.; Ramos-Ibarra, J.; Gutiérrez-González, P.; Pelayo-Ortiz, C.; Guatemala-Morales, G.; Arriola-Guevara, E. The use of response surface methodology to evaluate the fermentation conditions in the production of tepache. Rev. Mex. Ing. Qum. 2013, 12, 19–28. [Google Scholar]
- Moreno-Terrazas, R.; Reyes-Morales, H.; Huerta-Ochoa, S.; Guerrero-Legarreta, I.; Vernon-Carter, E.J. Note. Consumer awareness of the main sensory attributes of tepache, a traditional fermented fruit beverage. Food Sci. Technol. Int. 2001, 7, 411–415. [Google Scholar] [CrossRef]
- Velázquez-Quiñones, S.E.; Moreno-Jiménez, M.R.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Álvarez, S.A.; Rosales-Villarreal, M.C.; Rocha-Guzmán, N.E. Apple Tepache Fermented with Tibicos: Changes in Chemical Profiles, Antioxidant Activity and Inhibition of Digestive Enzymes. J. Food Process. Preserv. 2001, 45, e15597. [Google Scholar]
- Rodríguez-Lerma, G.K.; Gutiérrez-Moreno, K.; Cárdenas-Manríquez, M.; Botello-Álvarez, E.; Jiménez-Islas, H.; Rico-Martínez, R.; Navarrete-Bolaños, J.L. Microbial ecology studies of spontaneous fermentation: Starter culture selection for prickly pear wine production. J. Food Sci. 2011, 76, 6. [Google Scholar] [CrossRef]
- Lappe-Oliveras, P.; Moreno-Terrazas, R.; Arrizón-Gaviño, J.; Herrera-Suárez, T.; García-Mendoza, A.; Gschaedler-Mathis, A. Yeasts associated with the production of Mexican alcoholic non-distilled and distilled Agave beverages. FEMS Yeast Res. 2008, 8, 1037–1052. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Terrazas, R. Determinación de las Características Microbiológicas, Bioquímicas Fisicoquímicas y Sensoriales para la Estandarización del Procesode Elaboración del Tepache. Iztapalapa, México. Ph.D. Thesis, Universidad Autónoma Metropolitana, Mexico City, Mexico, 2005. [Google Scholar]
- Kirchmayr, M.; Segura, L.E.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; de la Rosa, M.; Gschaedler Mathis, A. Impact of envi-ronmental conditions and process modifications on microbial diversity, fermentation efficiency and chemical profile during the fermentation of Mezcal in Oaxaca. LWT—Food Sci. Technol. 2017, 79, 160–169. [Google Scholar] [CrossRef]
- Páez-Lerma, J.B.; Arias-García, A.; Rutiaga-Quiñones, O.M.; Barrio, E.; Soto-Cruz, N.O. Yeasts Isolated from the Alcoholic Fermentation of Agave duranguensis during Mezcal Production. Food Biotechnol. 2013, 27, 342–356. [Google Scholar] [CrossRef]
- Pérez, E.; González-Hernández, J.C.; Chávez-Parga, M.C.; Cortés-Penagos, C. Caracterización fermentativa de levaduras productoras de etanol a partir de jugo de Agave cupreata en la elaboración de mezcal. Rev. Mex. Ing. Quím. 2013, 12, 451–461. [Google Scholar]
- Walker, G.M.; Lappe-Oliveras, P.; Moreno-Terrazas, R.; Kirchmayr, M.; Arellano-Plaza, M.; Gschaedler-Mathis, A.C. Chapter 16 Yeasts Associated with the Production of Distilled Alcoholic Beverages. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer Nature: New York, NY, USA, 2019; pp. 478–515. [Google Scholar]
- Narváez-Zapata, J.A.; Rojas-Herrera, R.A.; Rodríguez-Luna, I.C.; Larralde-Corona, C.P. Culture-Independent Analysis of Lactic Acid Bacteria Diversity Associated with Mezcal Fermentation. Curr. Microbiol. 2010, 61, 444–450. [Google Scholar] [CrossRef]
- Castillo-Morales, M.; Wacher-Rodarte, C.; Hernández-Sánchez, H. Preliminary studies on chorote a tradicional Mexican fer-mented product. World J. Microbiol. Biotechnol. 2005, 21, 293–296. [Google Scholar] [CrossRef]
- Bernard Menna, A.; Lozano Cortés, M. “Las Bebidas Sagradas Mayas: El Balché Y El Saká.” Sincronía. Available online: http://sincronia.cucsh.udg.mx/mennacortes03.html (accessed on 16 June 2021).
- Aguirre-Dugua, X.; Pérez-Negrón, E.; Casas, A. Phenotypic differentiation between wild and domesticated varieties of Crescentia cujeteL. and culturally relevant uses of their fruits as bowls in the Yucatan Peninsula, Mexico. J. Ethnobiol. Ethnomed. 2013, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Chuchiak, J.F., IV. “It is Their Drinking That Hinders Them”: Balché and the Use of Ritual Intoxicants among the Colonial Yucatec Maya. Estud. Cult. Maya 2013, 24, 1550. [Google Scholar]
- Aroche, D.S. Con el diablo adentro. El consumo medicinal y ritual del balche’entre los mayas de Yucatán visto desde una perspectiva etnohistórica. Hist. Conoc. Hist. Clave Digit. 2016, 10, 42–55. [Google Scholar]
- Cox-Tamay, L.D.; Yamasaki, E.; Heredia-Campos, E.B. Plantas Utilizadas En La Ceremonia Maya: Ch’a Cháak. Desde El Herbario CICY 2016, 8, 167. Available online: https://www.academia.edu/29506808/Plantas_utilizadas_en_la_Ceremonia_Maya_Cha_ch%C3%A1 (accessed on 2 February 2021).
- Menezes, C.; Vollet-Neto, A.; Contrera, F.A.F.L.; Venturieri, G.C.; Imperatriz-Fonseca, V.L. The role of useful microorganisms to stingless bees and stingless beekeeping. In Pot-Honey; Springer: New York, NY, USA, 2013; pp. 153–171. [Google Scholar]
- Quintero-Salazar, B.; Bernáldez Camiruaga, A.I.; Dublán-García, O.; Barrera García, V.D.; Favila Cisneros, H.J. Consumo y conocimiento actual de una bebida fermentada tradicional en Ixtapan del Oro, México: La sambumbia. Alteridades 2012, 22, 115–129. [Google Scholar]
- Wacher Rodarte, C. La biotecnología alimentaria antigua: Los alimentos fermentados. Rev. Digit. Univ. 2014, 15, 121–136. [Google Scholar]
- Romero, E.B.S. El Sjendechjø: Bebida Mazahua de Maíz Fermentado; Consejo Estatal para el Desarrollo Integral de los Pueblos Indígenas del Estado de México: Mexico City, Mexico, 1995. [Google Scholar]
- Lorence-Quiñones, A.; Wacher-Rodarte, C.; Quintero-Ramírez, R. Cereal fermentations in Latin American countries. FAO Agric. Serv. Bull. 1999, 138, 99–114. [Google Scholar]
- Unai. “Sendechó, la Cerveza Precolombina”. 2019. Available online: https://www.delgranoalacopa.com/sendecho-la-cerveza-precolombina/ (accessed on 30 April 2021).
- De la Fuente-Salcido, N.M.; Castañeda-Ramírez, J.C.; García-Almendárez, B.E.; Bideshi, D.K.; Salcedo-Hernández, R.; Bar-boza-Corona, J.E. Isolation and characterization of bacteriocinogenic lactic bacteria from M-Tuba and Tepache, two traditional fermented beverages in México. Food Sci. Nutr. 2015, 3, 434–442. [Google Scholar] [CrossRef]
- Flores-Gallegos, A.; Vázquez-Vuelvas, O.; López-López, L.; Sainz-Galindo, A.; Ascacio-Valdes, J.; Aguilar, C.N.; Rodriguez-Herrera, R. Tuba, a Fermented and Refreshing Beverage from Coconut Palm Sap. In Non-Alcoholic Beverages; Woodhead Publishing: Sawston, UK, 2019; pp. 163–184. [Google Scholar]
- Coutiño, B.; Rodríguez, R.; Belmares, R.; Aguilar, C.; Ruelas, X. Selección de la bebida “taberna” obtenida de la palma Acro-comia aculeata y análisis químico proximal. Multiciencias 2015, 15, 397–409. [Google Scholar]
- Available online: https://www.gob.mx/agricultura|jalisco/articulos/alcanza-tequila-ventas-por-mas-de-568-millones-de-dolares-en-primer-semestre-del-ano-138868 (accessed on 12 February 2021).
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2020, 10, 13. [Google Scholar] [CrossRef]
- Wahyuni, N.L.; Sunarharum, W.B.; Muhammad, D.R.A.; Saputro, A.D. Formation and development of flavour of cocoa (Theobroma cacao L.) cultivar Criollo and Forastero: A review. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012078. [Google Scholar] [CrossRef]
- De Filippis, F.; Pasolli, E.; Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 2020, 44, 454–489. [Google Scholar] [CrossRef] [PubMed]
- Kisan, B.S.; Kumar, R.; Ashok, S.P.; Sangita, G. Probiotic foods for human health: A review. J. Pharmacogn. Phytochem. 2019, 8, 967–971. [Google Scholar]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołożyn-Krajewska, D. Trends and possibilities of the use of probiotics in food production. In Alternative and Replacement Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 65–94. [Google Scholar]
- Homayouni, A.; Alizadeh, M.; Alikhah, H. Functional dairy probiotic food development: Trends, concepts, and products. In Immunology and Microbiology “Probiotics”; IntechOpen: London, UK, 2012. [Google Scholar]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Aguilar, C.N. Food waste and byproducts: An opportunity to minimize malnutrition and hunger in developing countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Rad, A.H.; Abbasi, A.; Kafil, H.S.; Ganbarov, K. Potential Pharmaceutical and Food Applications of Postbiotics: A Review. Curr. Pharm. Biotechnol. 2020, 21, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Cortes, M.S.; Verdugo Valdez, A.G.; Lopez Zuñiga, E.J.; Vela Gutierrez, G. El Atole Agrio: Su Presencia y Modo de Preparación en la Cultura Alimentaria de Chiapas; UNICACH: Mexico City, Mexico, 2019. [Google Scholar]
- Bueno López, B.P. Evaluación del Potencial Probiótico de Bacterias Ácido Lácticas (BAL), Aisladas de una Bebida Fermentada Tradicional de Chiapas (Taberna). Doctoral Dissertation, Universidad de Ciencias y Artes de Chiapas, Tuxtla Gutiérrez, Chiapas, Mexico, 2013. [Google Scholar]
- Trujillo Jiménez, C.A. Evaluación de la capacidad productiva de etanol utilizando levaduras aisladas de agave y taberna. Doctoral dissertation, Universidad de Ciencias y Artes de Chiapas, Tuxtla Gutiérrez, Chiapas, Mexico, 2013. [Google Scholar]
- Alcántara-Hernández, R.J.; Rodríguez-Álvarez, J.A.; Valenzuela-Encinas, C.; Gutiérrez-Miceli, F.A.; Castañón-González, H.; Marsch, R.; Dendooven, L. The bacterial community in ‘taberna’a traditional beverage of Southern Mexico. Lett. Applied Microbiol. 2010, 51, 558–563. [Google Scholar] [CrossRef]
- Ambrocio Ríos, J.A. Ecología de Levaduras Asociadas a la Taberna, Bebida Extraída de la Palma de Coyol (Acrocomia aculeata (Jacq.) Lodd. ex Mart). Master’s Thesis, Universidad de Ciencias y Artes de Chiapas, Tuxtla Gutiérrez, Chiapas, Mexico, 2018. [Google Scholar]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Pakuwal, E.; Manandhar, P. Production of Rice Based Alcoholic Beverages and their Quality Evaluation. J. Food Sci. Technol. Nepal 2020, 12, 37–48. [Google Scholar] [CrossRef]
- Cano, A.N.H.; Suárez, M.E. Ethnobiology of algarroba beer, the ancestral fermented beverage of the Wichí people of the Gran Chaco I: A detailed recipe and a thorough analysis of the process. J. Ethn. Foods 2020, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Daryaei, H.; Coventry, J.; Versteeg, C.; Sherkat, F. Combined pH and high hydrostatic pressure effects on Lactococcus start-er cultures and Candida spoilage yeasts in a fermented milk test system during cold storage. Food Microbiol. 2010, 27, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter Cultures for Sparkling Wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces commercial starter cultures: Scientific trends, recent patents and innovation in the wine sector. Recent Pat. Food Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Valadez-Blanco, R.; Bravo, G.; Santos, N.; Velasco, S.; Montville, T. The artisanal production of pulque, a traditional beverage of the Mexican highlands. Probiotics Antimicrob. Proteins 2012, 4, 140–144. [Google Scholar] [CrossRef]
- Rizo, J.; Rogel, M.A.; Guillén, D.; Wacher, C.; Martinez-Romero, E.; Encarnación, S.; Rodríguez-Sanoja, R. Nitrogen fixation n pozol, a traditional fermented beverage. Appl. Environ. Microbiol. 2020, 86, e00588-20. [Google Scholar] [CrossRef]
- Nuñez-Guerrero, M.E.; Salazar-Vázquez, E.; Páez-Lerma, J.B.; Rodríguez-Herrera, R.; Soto-Cruz, N.O. Physiological characterization of two native yeasts in pure and mixed culture using fermentations of agave juice. Int. J. Agric. Nat. Resour. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Escalante-Minakata, P.; Blaschek, H.; de la Rosa, A.B.; Santos, L.; De León-Rodríguez, A. Identification of yeast and bacteria involved in the mezcal fermentation of Agave salmiana. Lett. Appl. Microbiol. 2008, 46, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Uribe, J.A.; Figueroa, L.M.; Martín-del-Campo, S.T.; Escalante, A. Pulque. In Fermented Foods in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2017; pp. 543–556. [Google Scholar]
- Jakočiūnas, T.; Jensen, M.K.; Keasling, J. CRISPR/Cas9 advances engineering of microbial cell factories. Metab. Eng. 2016, 34, 44–59. [Google Scholar] [CrossRef] [Green Version]
- Jakočiūnas, T.; Bonde, I.; Herrgård, M.; Harrison, S.J.; Kristensen, M.; Pedersen, L.E.; Jensen, M.K.; Keasling, J.D. Multiplex metabolic pathway engineering using CRISPR/Cas9 in Saccharomyces cerevisiae. Metab. Eng. 2015, 28, 213–222. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, J.; Norville, J.; Mali, P.; Rios, X.; Aach, J.; Church, G.M. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013, 41, 4336–4343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, F.; Krogerus, K.; Castillo, S.; Ortiz-Julien, A.; Dequin, S.; Gibson, B. Exploring the potential of Saccharomyces eubayanus as a parent for new interspecies hybrid strains in winemaking. FEMS Yeast Res. 2017, 17, 17. [Google Scholar] [CrossRef]
- Nikulin, J.; Vidgren, V.; Krogerus, K.; Magalhães, F.; Valkeemäki, S.; Kangas-Heiska, T.; Gibson, B. Brewing potential of the wild yeast species Saccharomyces paradoxus. Eur. Food Res. Technol. 2020, 246, 2283–2297. [Google Scholar] [CrossRef]
- Wagner, J.M.; Alper, H.S. Synthetic biology and molecular genetics in non-conventional yeasts: Current tools and future advances. Fungal Genet. Biol. 2016, 89, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Xie, W.; Li, X.; Cai, G.; Lu, J.; Xie, G. Metabolic engineering of Saccharomyces cerevisiae using the CRISPR/Cas9 system to minimize ethyl carbamate accumulation during Chinese rice wine fermentation. Appl. Microbiol. Biotechnol. 2020, 104, 4435–4444. [Google Scholar] [CrossRef]
- Vigentini, I.; Gebbia, M.; Belotti, A.; Foschino, R.; Roth, F. CRISPR/Cas9 System as a Valuable Genome Editing Tool for Wine Yeasts with Application to Decrease Urea Production. Front. Microbiol. 2017, 8, 2194. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Suarez, R.; Morales Chavez, L.A.; Galvez Mariscal, A. Importance of mexican maize landraces in the national diet: An essential review. Rev. Fitotec. Mex. 2013, 36, 275–283. [Google Scholar]
- Valerino-Perea, S.; Lara-Castor, L.; Armstrong, M.E.G.; Papadaki, A. Definition of the Traditional Mexican Diet and Its Role in Health: A Systematic Review. Nutrients 2019, 11, 2803. [Google Scholar] [CrossRef] [Green Version]
- Delgado, Á.A. La mujer en la cosmovisión y ritualidad rarámuri. Bol. Antropol. Univ. Antioq. 2007, 21, 41–63. [Google Scholar]
- Delgado, Á.A.; Gómez, G.A. The rarámuri race as a metaphor of cultural resistance. J. Hum. Sport Exerc. 2009, 4, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.L.; Klaus, H.D. Diet, Nutrition, and Foodways on the North Coast of Peru; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lertzman, K. The Paradigm of Management, Management Systems, and Resource Stewardship. J. Ethnobiol. 2009, 29, 339–358. [Google Scholar] [CrossRef] [Green Version]
- Salomon, A.K.; Lertzman, K.; Brown, K.; Wilson, K.B.; Secord, D.; McKechnie, I. Democratizing conservation science and practice. Ecol. Soc. 2018, 23, 23. [Google Scholar] [CrossRef]
- Ham, P.F. Infant mortality in the indigenous population: Backwardness and contrasts. Demos 1993, 6, 12–13. [Google Scholar]
- Serrano Carreto, E.; Embriz Osorio, A.; Fernández Ham, P. Indicadores Socioeconómicos de los Pueblos Indígenas de México; Instituto Nacional Indígenista: Mexico City, Mexico, 2002. [Google Scholar]
- Lartigue, F.; Quesnel, A. La Población Indígena Entre los Enfoques de Política Pública y las Categorías Antropodemográficas; Alteridades, CIESAS: Mexico City, Mexico, 2003; pp. 11–34. [Google Scholar]
- Barbary, O. Social Inequalities and Indigenous Populations in Mexico: A Plural Approach. In Social Statistics and Ethnic Diversity; Springer: Cham, Switzerland, 2015; pp. 209–228. [Google Scholar]
- Cerjak, M.; Haas, R.; Brunner, F.; Tomić, M. What motivates consumers to buy traditional food products? Evidence from Croatia and Austria using word association and laddering interviews. Br. Food J. 2014, 116, 1726–1747. [Google Scholar] [CrossRef]
- Vanhonacker, F.; Van Loo, E.J.; Gellynck, X.; Verbeke, W. Flemish consumer attitudes towards more sustainable food choices. Appetite 2013, 62, 7–16. [Google Scholar] [CrossRef]
- Almansouri, M.; Verkerk, R.; Fogliano, V.; Luning, P.A. Exploration of heritage food concept. Trends Food Sci. Technol. 2021, 111, 790–797. [Google Scholar] [CrossRef]
- Senos, R.; Lake, F.K.; Turner, N.; Martinez, D. Traditional ecological knowledge and restoration practice. In Restoring the Pacific Northwest: The Art and Science of Ecological Restoration in Cascadia; Apostol, D., Sinclair, M., Eds.; Island Press: Washington, DC, USA, 2006; Chapter 17; pp. 393–426. [Google Scholar]
- Kuhnlein, H.V.; Receveur, O. Dietary change and traditional food systems of indigenous peoples. Annu. Rev. Nutr. 1996, 16, 417–442. [Google Scholar] [CrossRef]
- Turner, N.J.; Turner, K.L. Traditional Food Systems, Erosion and Renewal in Northwestern North America. Indian J. Tradit. Knowl. 2007, 6, 57–68. [Google Scholar]
- Quave, C.; Pieroni, A. Fermented Foods for Food Security and Food Sovereignty in the Balkans: A Case Study of the Gorani People of Northeastern Albania. J. Ethnobiol. 2014, 34, 28–43. [Google Scholar] [CrossRef]
- Posey, D.A. Biodiversity, genetic resources and indigenous peoples in Amazonia: (Re) discovering the wealth of traditional resources of Native Amazonians. In Amazonia at the Crossroads; Oxford University Press: Oxford, UK, 2000; pp. 188–204. [Google Scholar]
- Mauro, F.; Hardison, P.D. Traditional knowledge of indigenous and local communities: International debate and policy initiatives. Ecol. Appl. 2000, 10, 1263–1269. [Google Scholar] [CrossRef]
- Drahos, P. Developing Countries and International Intellectual Property Standard-Setting. J. World Intellect. Prop. 2005, 5, 765–789. [Google Scholar] [CrossRef]
- Hafstein, V.T. The Making of Intangible Cultural Heritage: Tradition and Authenticity, Community and Humanity; University of California: Berkeley, CA, USA, 2004. [Google Scholar]
- Alexiades, M.N. Ethnobotany in the third millennium: Expectations and unresolved issues. Delpinoa 2003, 45, 15–28. [Google Scholar]
- Rocha, J.A.; Boscolo, O.H.; Fernandes, L.R. Ethnobotany: A instrument for valorisation and identification of potential for the protection of traditional knowledge. Interações Campo Grande 2015, 16, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Mintz, S.W.; Du Bois, C.M. The Anthropology of Food and Eating. Annu. Rev. Anthr. 2002, 31, 99–119. [Google Scholar] [CrossRef] [Green Version]
- Ingold, T. The Perception of the Environment: Essays on Livelihood, Dwelling and Skill; Routledge: London, UK, 2002. [Google Scholar]
- Ellen, R.F.; Fischer, M.D. Introduction: On the Concept of Cultural Transmission; Berhahn Books: New York, NY, USA; Oxford, UK, 2013; pp. 1–54. [Google Scholar]
- Pelto, G.H.; Vargas, L.A. Perspectives in dietary change. Ecol. Food Nutr. 1992, 27, 159–161. [Google Scholar] [CrossRef]
- Marshall, E.; Mejia, D. Traditional Fermented Food and Beverages for Improved Livelihoods; FAO Diversification Booklet; FAO: Rome Italy, 2011; p. 21. [Google Scholar]
- Pai, J.S. Applications of microorganisms in food biotechnology. Indian J. Biotech. 2003, 2, 382–386. [Google Scholar]
- Alexiades, M. The cultural and economic globalisation of traditional environmental knowledge systems. In Landscape, Process and Power: Reevaluating Traditional Environmental Knowledge; Berghahn Books: New York, NY, USA; Oxford, UK, 2009; pp. 68–90. [Google Scholar]
- Cunningham-Sabo, L.D.; Davis, S.M.; Koehler, K.M.; Fugate, M.L.; DiTucci, J.A.; Skipper, B.J. Food preferences, practices, and cancer-related food and nutrition knowledge of southwestern American Indian youth. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1996, 78, 1617–1622. [Google Scholar]
- Reyes-García, V.; Menendez-Baceta, G.; Aceituno-Mata, L.; Acosta-Naranjo, R.; Calvet-Mir, L.; Domínguez, P.; Pardo-de-Santayana, M. From famine foods to delicatessen: Interpreting trends in the use of wild edible plants through cultural eco-system services. Ecol. Econ. 2015, 120, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Holguín Balderrama, J. Yocogihua: La Última Fábrica de Aguardiente Alamense. Available online: https://infocajeme.com/general/2018/03/yocogihua-la-ultima-fabrica-de-aguardiente-alamense/ (accessed on 26 March 2018).
- Nabhan, G.P.; Suro Pineda, D. Agave Spirit World Past, Present and Future of Mezcals and Their Kin; W.W. Norton: New York, NY, USA, 2021. [Google Scholar]




| Beverages | Main Substrate | Main Microorganisms Recorded in the Literature | Cultural Groups Associated | Literature |
|---|---|---|---|---|
| Pozol | Zea mays (grains) | Bacteria: Aerobacter, Acetobacter, Achromobacter, Agrobacterium, Alcaligenes, Bacillus, Bifidobacterium, Clostridium, Enterobacter, Enterococcus, Escherichia, Exiguabacterium, Klebsiella, Kosakonia Lactobacillus, Lactococcus, Leuconostoc, Paracolobactrum, Pediococcus, Pseudomonas, Propionibacterium, Streptococcus, Weissella. Yeasts: Candida, Cyberlindera, Debaryomyces, Kluyveromyces, Galactomyces, Meyerozyma, Pichia, Rhodotorula, Trichosporon. Fungi: Cladosporium, Monilia, Mucor, Phoma, Penicillium. | Chol, Chontal, Lacandon, Mam, Maya, Tojolabal, Tzeltal, Tzotzil, Zapotec, Zoque, Mestizo | [52,53,54,55,61,68,69,70] [71,72,73,74,75,76,77,78,79] |
| Atole agrio | Zea mays (grains) | Bacteria: Acetobacter, Aerococcus, Bacillus, Enterococcus, Clostridium, Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Streptococcus, Weissella. Yeasts: Candida, Cryptococcus, Clavispora, Debaryomyces, Hanseniaspor, Pichia Saccharomycesa. Fungi: Aspergillus, Fusarium, Penicillium. | Maya, Mazatec, Nahua, Purepecha, Totonac, Tzeltal, Tzotzil, Wixarika, Mestizo | [58,72,73,80,81,82,83] |
| Saká | Zea mays (grains) | ND | Maya, Tzotzil, Tzeltal | [84] |
| Tejuino | Zea mays (grains) | Bacteria: Acetobacter, Bacillus, Brochothrix, Chyseobacterium, Kurthia, Lactobacillus, Leuconostoc, Pantoea, Pseudomoonas, Strotococcus, Weissella. Yeasts: Candida, Galactomyces, Lachancea, Meyerozyma, Saccharomyces, Wickehamomyces.Fungi. Aspergillus, Penicillium | Mestizo | [85,86] |
| Tesgüino | Zea mays (grains) | Bacteria: Bacillus, Lactobacillus, Bacillus, Leuconostoc, Pediococcus, Streptococcus. Yeasts: Brettanomyces, Candida, Clavispora, Cryptococcus, Kluyveromyces, Lachancea, Metschnikowia, Meyerozyma, Pichia, Saccharomyces, Wicherhamomyces. Fungi: Aspergillus, Penicillium. | Guajiro, Pame, Pima, Tarahumara, Tepehuan, Tubar, Wixarika, Yaqui, Zapotec | [46,47,48,49,87,88,89,90,91,92] |
| Pulque | Agave spp. (sap) | Bacteria: Acetobacter, Acetobacterium, Acinetobacter, Acrobacter, Adlercreutzia, Ardescatena, Bacillus, Commensalibacter, Citrobacter, Cellulomonas, Cellulosimicrobium, Chelativorum, Chryseobacterium, Chryseomonas, Clostridium, Comensalbacter, Corynebacterium, Devosia, Dysgonomonas, Enterobacter, Erwinia, Escherichia, Euzebia, Flavobacterium, Fructobacillus, Gluconobacter, Hafnia, Halomicronema, Kluyvera, Klebsiella, Kokuria, Komagataeibacter, Lactobacillus, Lactococcus, Luteomicrobium, Leuconostoc, Marivitia, Macrococcus, Mesorhizobium, Micrococcus, Microbacterium, Micrococcus, Novosphingobium, Providencia, Pediococcus, Pseudomonas, Rhodobacter, Rhodovulum, Ruminococcus, Sacrcina, Salinibacterim. Sarcandra, Serratia, Sphaerotilus, Sphingomonas, Sphingopyxis, Streptococcus, Streptomyces, Sulfuropirillum, Synechococcus, Tanticharoenia, Trochococcus, Weissella, Zymomonas. Yeasts: Bullera, Candida, Clavispora, Cryptococcus, Cystofilobasidium, Debaryomyces, Dekkera, Galactomyces, Hanseniaspora, Kazachstania, Kluyveromyces, Lipomyces, Meyerozyma, Pichia, Rhodotorula, Saccharomyces, Schwanniomyces. Torulaspora, Westerdykella, Wickerhamomyces, Zygosaccharomyces. Fungi: Aureobasidium. Aspergillus, Cladosporium. Penicillium, Rhizopus. | Hñähñu, Ixcatec, Mazahua, Mixtec, Nahua, Ngiwa, Purhepecha, Triqui, Zapotec, Mestizo | [23,45,46,47,48,93,94,95,96,97] |
| Tuba | Cocos nucifera (sap) | Bacteria: Bacillus, Cronobacter, Enterococcus, Erwinia, Fructobacillus, Gluconacetobacter, Klebsiella, Lactobacillus, Lactococcus, Leuconostoc, Micrococcus, Serratia, Sphingomonas, Vibrio, Zymomonas. Yeasts: Candida, Cryptococcus, Hanseniaspora, Saccharomyces. | Mestizo | [96,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115] |
| Taberna | Acrocomia acuelata (sap) | Bacteria: Aerobacter, Acetobacter, Bacillus, Brevundimonas, Citrobacter, Enterobacter, Enterococcus, Fructobacillus, Gluconobacter, Klebsiella, Kluyvera, Lactobacillus, Lactococcus, Pantoea, Sphingomonas, Zymomonas. Yeasts: Candida, Hanseniapora, Issatchenkia, Kazachstania. Meyerozyma, Pichia. Rhodotorula, Saccharomyces, Schizosaccharomyces | Zapotec, Mestizo | [98,99,100] |
| Tepache | Ananas comosus (fruit) | Bacteria: Acetobacter, Acinetobacter, Bacillus,
Escherichia, Enterobacter, Enterococcus, Gluconobacter, Klebsiella, Lactobacillus, Lactococcus, Leuconostoc, Micrococcus, Pediococcusa, Weissella. Yeasts: Candida, Cryptococcus, Hanseniaspora, Meyerozyma, Pichia, Rhodotorula, Saccharomyces.Fungi: Penicillium. | Mestizo | [49,50,116,117,118] |
| Colonche | Opuntia spp. (fruits), Pacchycerus, Stenocereus | Bacteria: Enterococcus, Lactobacillus, Leuconoctoc, Pediococcus, Weissella.Yeasts: Candida, Hanseniaspora, Pichia, Saccharomyces. | Chichimecan groups, Pame, Zapotec, Mestizo | [22,50,52,119] |
| Mescal | Agave spp. | Bacteria: Acetobacter, Acinetobacter, Acetobacterium, Bacillus, Citrobacter, Enterobacter, Erwinia, Chryseobacterium, Gluconobacter, Kluyvera, Kokuria, Komagataeibacter, Lactobacillus, Lactococcus, Leuconostoc, Microbacterium, Providencia, Oenococcus, Pediococcus, Pseudomonas Serratia, Weissella, Zymomonas. Yeasts: Candida, Citeromyces, Clavispora, Cryptococcus, Debaryomyces, Dekkera, Diutinia, Hanseniaspora, Issatchenkia, Kazachstania, Kluyveromyces, Meyerozyma, Millerozyma,Naganishia, Ogataea, Pichia, Pseudozyma, Rhodosporidiobolus, Rhodotorula, Saccharomyces, Saturnispora, Schizosaccharomyces Sporidiobolus, Torulaspora, Trichosporon, Wickerhamomyces, Yamadazyma, Zygosaccharomyces. | Cahiti, Guasave, Ixcatec, Pima, Tepehuan, Warohiro, Wixarika, Mestizo | [116,120,121,122,123,124,125,126] |
| Chorote | Zea mayz (grains) Theobroma cacao rosted beans), | Fructobacillus, Lactobacillus, Leuconostoc, Gluconacetobacter, Sphingomonas, Vibrio, | Maya, Mestizo | [127] |
| Balché | Lonchocarpus spp. (bark and flowers) and honeybee | Yeasts: Saccharomyces. | Lacandon, Maya | [84,128,129,130,131,132,133] |
| Pox | Saccharum officinarum and Zea mays (stems) | ND | Chol, Tzeltal, Tzotzil Mestizo | [53] |
| Sambudia | Ananas comosus (fruit) | ND | Mazahua, Mestizo | [134,135,136] [48,49,50,137,138,139,140,141] |
| Sendechó | Zea mays (grains) and Capsicum sp. | Bacteria: Acetobacter, Bacillus, Enterococcus, Klebsiella, Kocuria, Lactobacillus, Leuconostoc, Micrococcus, Pediococcus, Pseudomionas, Staphylococcus, Zymomonas.Yeasts: Candida, Clavispora, Cryptococcus, Galactomyces, Kluyveromyces, Rhodotorula, Saccharomyces,
Torulaspora, Wickerhamomyces, Zygosaccharomyces. Fungi: Aureobasidium, Cladosporium, Epicoccum, Fusarium, Paecilomyces, Penicillum, Phima, Sclerotium, Verticillium. | Mazahua, Hñähñu |
| Network | Connectance | Links per Species | Niche Overlap | Mean of Shared Partners |
|---|---|---|---|---|
| Plant substrates | 0.085 | 1.29 | 0.20 | 0.31 |
| Microorganisms | 0.23 | 1.75 | 0.99 | 7.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Linares, C.; Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Islas, L.A.; Lappe-Oliveras, P.; Nabhan, G.P.; Torres-García, I.; Vallejo, M.; Casas, A. Traditional Fermented Beverages of Mexico: A Biocultural Unseen Foodscape. Foods 2021, 10, 2390. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102390
Ojeda-Linares C, Álvarez-Ríos GD, Figueredo-Urbina CJ, Islas LA, Lappe-Oliveras P, Nabhan GP, Torres-García I, Vallejo M, Casas A. Traditional Fermented Beverages of Mexico: A Biocultural Unseen Foodscape. Foods. 2021; 10(10):2390. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102390
Chicago/Turabian StyleOjeda-Linares, César, Gonzalo D. Álvarez-Ríos, Carmen Julia Figueredo-Urbina, Luis Alfredo Islas, Patricia Lappe-Oliveras, Gary Paul Nabhan, Ignacio Torres-García, Mariana Vallejo, and Alejandro Casas. 2021. "Traditional Fermented Beverages of Mexico: A Biocultural Unseen Foodscape" Foods 10, no. 10: 2390. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10102390