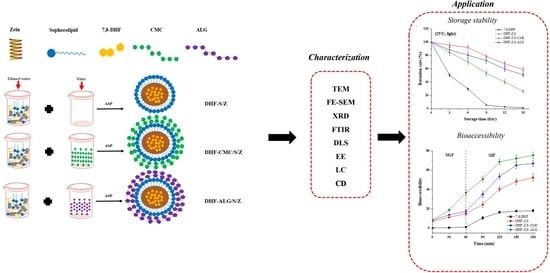
Development, Characterization, Stability and Bioaccessibility Improvement of 7,8-Dihydroxyflavone Loaded Zein/Sophorolipid/Polysaccharide Ternary Nanoparticles: Comparison of Sodium Alginate and Sodium Carboxymethyl Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Zein/Sophorolipid/Polysaccharide Ternary Nanoparticles Preparation
2.3. Polydispersity Index (PDI), Particle Size, Zeta Potential and Turbidity
2.4. Physical Stability of Ternary Nanoparticles
2.4.1. pH Influence
2.4.2. Ionic Strength Influence
2.5. Encapsulation of 7,8-DHF into Ternary Nanoparticles
2.6. Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.7. 7,8-DHF Loaded Ternary Nanoparticles Characterization
2.7.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.7.2. Circular Dichroism (CD) Spectrum
2.7.3. Differential Scanning Calorimetry (DSC)
2.7.4. X-ray Diffraction (XRD)
2.7.5. Transmission Electron Microscopy (TEM)
2.7.6. Field Emission Scanning Electron Microscope (FE-SEM)
2.8. Storage Stability of 7,8-DHF
2.9. In Vitro Simulated Gastrointestinal Digestion
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Polysaccharides on Ternary Nanoparticles Fabrication
3.2. CMC and ALG Prevents S/Z Ternary Nanoparticles Precipitation at pH = 4
3.3. Physical-Chemical Stability Study on Ternary Nanoparticles
3.3.1. Effect of pH
3.3.2. Effect of Ionic Strengths
3.4. Encapsulation of 7,8-DHF
3.5. Characterization of Loaded Nanoparticles
3.5.1. FTIR
3.5.2. CD Spectrum
3.5.3. DSC
3.5.4. XRD
3.6. Micromorphology
3.7. A Graphic Illustration for the Formation and Stability Mechanism of Nanoparticles
3.8. Storage Stability of 7,8-DHF
3.9. In Vitro Simulated Gastrointestinal Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andero, R.; Ressler, K.J. Fear extinction and bdnf: Translating animal models of ptsd to the clinic. Genes Brain Behav. 2012, 11, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, P.S.; Flamini, G.; Christodoulou, M.S.; Rodondi, G.; Vitalini, S.; Passarella, D.; Fico, G. Farinose alpine primula species: Phytochemical and morphological investigations. Phytochemistry 2014, 98, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E. A selective trkb agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.H.; Dai, C.F.; Chen, L.; Zhou, W.T.; Han, H.L.; Dong, Z.F. 7,8-dihydroxyflavone ameliorates motor deficits via suppressing α-synuclein expression and oxidative stress in the mptp-induced mouse model of parkinson’s disease. CNS Neurosci. Ther. 2016, 22, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytan, N.; Choi, J.; Carreras, I.; Crabtree, L.; Nguyen, B.; Lehar, M.; Blusztajn, J.K.; Jenkins, B.G.; Dedeoglu, A. Protective effects of 7,8-dihydroxyflavone on neuropathological and neurochemical changes in a mouse model of alzheimer’s disease. Eur. J. Pharmacol. 2018, 828, 9–17. [Google Scholar] [CrossRef]
- Chan, C.B.; Tse, M.C.L.; Liu, X.; Zhang, S.; Schmidt, R.; Otten, R.; Liu, L.; Ye, K. Activation of muscular trkb by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem. Biol. 2015, 22, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, Z.; Zhang, Z.; Liu, X.; Kang, S.S.; Zhang, Y.; Ye, K. The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 578–583. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yao, W.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Hashimoto, K. Comparison of ketamine, 7,8-dihydroxyflavone, and ana-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 2015, 232, 4325–4335. [Google Scholar] [CrossRef]
- Liu, C.; Chan, C.B.; Ye, K. 7,8-dihydroxyflavone, a small molecular trkb agonist, is useful for treating various bdnf-implicated human disorders. Transl. Neurodegener. 2016, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xue, F.; Xia, G.; Zhao, Z.; Chen, C.; Li, Y.; Zhang, Y. Transepithelial transport mechanisms of 7,8-dihydroxyflavone, a small molecular trkb receptor agonist, in human intestinal caco-2 cells. Food Funct. 2019, 10, 5215–5227. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, G.; Zhao, Z.; Xue, F.; Gu, Y.; Chen, C.; Zhang, Y. 7,8-dihydroxyflavone nano-liposomes decorated by crosslinked and glycosylated lactoferrin: Storage stability, antioxidant activity, in vitro release, gastrointestinal digestion and transport in caco-2 cell monolayers. J. Funct. Foods 2020, 65, 103742. [Google Scholar] [CrossRef]
- Behjati, J.; Yazdanpanah, S. Nanoemulsion and emulsion vitamin d3 fortified edible film based on quince seed gum. Carbohyd. Polym. 2021, 262, 117948. [Google Scholar] [CrossRef]
- Niu, F.; Hu, D.; Gu, F.; Du, Y.; Zhang, B.; Ma, S.; Pan, W. Preparation of ultra-long stable ovalbumin/sodium carboxymethylcellulose nanoparticle and loading properties of curcumin. Carbohyd. Polym. 2021, 271, 118451. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Li, R.; Peng, S.; Ruan, R.; Li, J.; Liu, W. Fabrication of caseinate stabilized thymol nanosuspensions via the ph-driven method: Enhancement in water solubility of thymol. Foods 2021, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, C.; Shi, J.; Ni, F.; Shen, Q.; Xie, H.; Wang, K.; Lei, Q.; Fang, W.; Ren, G. The regulation of sodium alginate on the stability of ovalbumin-pectin complexes for vd3 encapsulation and in vitro simulated gastrointestinal digestion study. Food Res. Int. 2021, 140, 110011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, X.; Li, Y.; Yin, X.; Subirade, M.; Zhou, P.; Liang, L. The folic acid/β-casein complex: Characteristics and physicochemical implications. Food Res. Int. 2014, 57, 162–167. [Google Scholar] [CrossRef]
- Chen, S.; Ma, Y.; Dai, L.; Liao, W.; Zhang, L.; Liu, J.; Gao, Y. Fabrication, characterization, stability and re-dispersibility of curcumin-loaded gliadin-rhamnolipid composite nanoparticles using ph-driven method. Food Hydrocolloid. 2021, 118, 106758. [Google Scholar] [CrossRef]
- Xiao, Y.; Ho, C.; Chen, Y.; Wang, Y.; Wei, Z.; Dong, M.; Huang, Q. Synthesis, characterization, and evaluation of genistein-loaded zein/carboxymethyl chitosan nanoparticles with improved water dispersibility, enhanced antioxidant activity, and controlled release property. Foods 2020, 9, 1604. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, G.; Li, W.; Lv, L.; Zhang, Q. The role of ultrasound in the preparation of zein nanoparticles/flaxseed gum complexes for the stabilization of pickering emulsion. Foods 2021, 10, 1990. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Impact of microfluidization and thermal treatment on the structure, stability and in vitro digestion of curcumin loaded zein-propylene glycol alginate complex nanoparticles. Food Res. Int. 2020, 138, 109817. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; Mcclements, D.J.; Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for curcumin. Food Hydrocolloid. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Yu, Z.; Lin, K.; Yang, S.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Enhanced stability, structural characterization and simulated gastrointestinal digestion of coenzyme Q10 loaded ternary nanoparticles. Food Hydrocolloid. 2019, 94, 333–344. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Y.; Xu, Y.; Niu, F.; Li, Z.; Ba, C.; Jin, B.; Chen, G.; Li, X. One-step assembly of zein/caseinate/alginate nanoparticles for encapsulation and improved bioaccessibility of propolis. Food Funct. 2019, 10, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of curcumin in zein/caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocolloid. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, G.; Zhao, Z.; Xue, F.; Chen, C.; Zhang, Y. Formation, structural characterization, stability and in vitro bioaccessibility of 7,8-dihydroxyflavone loaded zein-/sophorolipid composite nanoparticles: Effect of sophorolipid under two blending sequences. Food Funct. 2020, 11, 1810–1825. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.; Kang, J.; Kong, Y. Dual-drug delivery system based on the hydrogels of alginate and sodium carboxymethyl cellulose for colorectal cancer treatment. Carbohyd. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Liu, C.; Zhang, S.; Xu, Y.; Wang, D. Fabrication and characterization of lutein-loaded nanoparticles based on zein and sophorolipid: Enhancement of water solubility, stability, and bioaccessibility. J. Agric. Food Chem. 2019, 67, 11977–11985. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Z.; Lin, K.; Sun, C.; Dai, L.; Yang, S.; Mao, L.; Yuan, F.; Gao, Y. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocolloid. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Wei, Y.; Zhan, X.; Mao, L.; Gao, Y. Formation and characterization of zein-propylene glycol alginate-surfactant ternary complexes: Effect of surfactant type. Food Chem. 2018, 258, 321–330. [Google Scholar] [CrossRef]
- Patel, A.R.; Ten-Hoorn, J.S.; Hazekamp, J.; Blijdenstein, T.B.; Velikov, K.P. Colloidal complexation of a macromolecule with a small molecular weight natural polyphenol: Implications in modulating polymer functionalities. Soft Matter 2013, 9, 1428–1436. [Google Scholar] [CrossRef]
- Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C.; Mcclements, D.J. Enhancement of curcumin bioavailability by encapsulation in sophorolipid-coated nanoparticles: An in vitro and in vivo study. J. Agric. Food Chem. 2018, 66, 1488–1497. [Google Scholar] [CrossRef]
- Yao, K.; Chen, W.; Song, F.; Mcclements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocolloid. 2018, 79, 262–272. [Google Scholar] [CrossRef]
- Hu, K.; Mcclements, D.J. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocolloid. 2015, 44, 101–108. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.; Wei, Y.; Sun, C.; Mao, L.; Gao, Y. Fabrication of zein and rhamnolipid complex nanoparticles to enhance the stability and in vitro release of curcumin. Food Hydrocolloid. 2018, 77, 617–628. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Liu, C.; Zhu, J.; Xu, Y.; Zhang, S.; Fan, M.; Zhang, D.; Zhang, Y.; Zhang, Z. Fabrication of stable zein nanoparticles by chondroitin sulfate deposition based on antisolvent precipitation method. Int. J. Biol. Macromol. 2019, 139, 30–39. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; Gao, Y. Binary complex based on zein and propylene glycol alginate for delivery of quercetagetin. Biomacromolecules 2016, 17, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yuan, P.; She, X.; Xia, Y.; Komarneni, S.; Xi, K.; Che, Y.; Yao, X.; Yang, D. Sustainable seaweed-based one-dimensional (1d) nanofibers as high-performance electrocatalysts for fuel cells. J. Mater. Chem. A 2015, 3, 14188–14194. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, L. Structural and functional properties of soy protein isolates modified by soy soluble polysaccharides. J. Agric. Food Chem. 2016, 64, 7275–7284. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Souza, B.W.; Teixeira, J.A.; Vicente, A.A. Effect of glycerol and corn oil on physicochemical properties of polysaccharide films—A comparative study. Food Hydrocolloid. 2012, 27, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, C.; Mcclements, D.J.; Gao, Y. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocolloid. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Xia, G.; Xue, F.; Chen, C.; Zhang, Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7,8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2020, 146, 179–192. [Google Scholar] [CrossRef]
- Liu, E.; Su, Z.; Yang, C.; Ji, Y.; Liu, B.; Meng, X. Fabrication, characterization and properties of dha-loaded nanoparticles based on zein and plga. Food Chem. 2021, 360, 129957. [Google Scholar] [CrossRef]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Assaf, A.; Thouand, G.; Le-Bail, A. Study of structural changes of gluten proteins during bread dough mixing by raman spectroscopy. Food Chem. 2021, 358, 129916. [Google Scholar] [CrossRef]
- Narwal, S.; Kumar, A.; Chaudhary, M.; Budhwar, V. Formulation of eutectic mixture of curcumin with salicylic acid for improving its dissolution profile. Res. J. Pharm. Technol. 2021, 14, 1875–1879. [Google Scholar]
- Jiang, F.; Yang, L.; Wang, S.; Ying, X.; Ling, J.; Ouyang, X.K. Fabrication and characterization of zein-alginate oligosaccharide complex nanoparticles as delivery vehicles of curcumin. J. Mol. Liq. 2021, 342, 116937. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Liu, F.; Peng, F.; Li, F.; Lou, X.; Jin, Y.; Wang, J.; Xu, H. Fabrication and characterization of zein-tea polyphenols-pectin ternary complex nanoparticles as an effective hyperoside delivery system: Formation mechanism, physicochemical stability, and in vitro release property. Food Chem. 2021, 364, 130335. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dai, L.; Gao, Y. Interaction and formation mechanism of binary complex between zein and propylene glycol alginate. Carbohyd. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef]
- Wang, W.; Liu, F.; Gao, Y. Quercetagetin loaded in soy protein isolate–κ-carrageenan complex: Fabrication mechanism and protective effect. Food Res. Int. 2016, 83, 31–40. [Google Scholar] [CrossRef]
- Liang, H.; Zhou, B.; He, L.; An, Y.; Lin, L.; Li, Y.; Liu, S.; Chen, Y.; Li, B. Fabrication of zein/quaternized chitosan nanoparticles for the encapsulation and protection of curcumin. RSC Adv. 2015, 5, 13891–13900. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Huang, J.; Dai, L.; Du, J.; Mcclements, D.J.; Mao, L.; Liu, J.; Gao, Y. Fabrication and characterization of layer-by-layer composite nanoparticles based on zein and hyaluronic acid for codelivery of curcumin and quercetagetin. ACS Appl. Mater. Inter. 2019, 11, 16922–16933. [Google Scholar] [CrossRef] [PubMed]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of essential oils in zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef] [PubMed]












| Zein: 7,8-DHF (w/w) | Colloidal Systems | EE (%) | LC (%) | Particle Size (nm) | PDI |
|---|---|---|---|---|---|
| Without 7,8-DHF | S/Z | - | 82.81 ± 0.61 a | 0.155 ± 0.010 a | |
| CMC/S/Z | - | 145.6 ± 0.75 c | 0.228 ± 0.006 b | ||
| ALG/S/Z | - | 178.2 ± 0.35 d | 0.266 ± 0.024 c | ||
| 5:1 | S/Z | 82.42 ± 3.72 a | 7.49 ± 0.22 a | 114.7 ± 3.01 b | 0.271 ± 0.016 c |
| CMC/S/Z | 88.63 ± 3.01 b | 7.38 ± 0.13 a | 177.4 ± 3.04 a | 0.363 ± 0.023 de | |
| ALG/S/Z | 84.15 ± 2.63 a | 7.01 ± 0.16 a | 214.3 ± 3.21 e | 0.394 ± 0.022 e | |
| 10:1 | S/Z | 98.21 ± 1.31 c | 4.68 ± 0.40 b | 106.9 ± 1.11 b | 0.201 ± 0.021 b |
| CMC/S/Z | 99.51 ± 0.24 c | 4.33 ± 0.12 b | 168.4 ± 3.62 dc | 0.334 ± 0.014 d | |
| ALG/S/Z | 98.71 ± 1.12 c | 4.29 ± 0.35 b | 200.1 ± 2.01 e | 0.352 ± 0.017 de |
| Sample | Content (%) | |||
|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turns | Unordered | |
| DHF-S/Z | 25.3 ± 0.23 | 25.8 ± 0.21 | 20.6 ± 0.19 | 28.3 ± 0.26 |
| DHF-CMC/S/Z | 24.7 ± 0.18 | 26.1 ± 0.17 | 20.1 ± 0.21 | 29.1 ± 0.20 |
| DHF-ALG/S/Z | 24.5 ± 0.16 | 26.6 ± 0.23 | 21.0 ± 0.26 | 27.9 ± 0.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Peng, J.; Wang, Y.; Wadhawan, D.; Wu, L.; Gao, X.; Sun, Y.; Xia, G. Development, Characterization, Stability and Bioaccessibility Improvement of 7,8-Dihydroxyflavone Loaded Zein/Sophorolipid/Polysaccharide Ternary Nanoparticles: Comparison of Sodium Alginate and Sodium Carboxymethyl Cellulose. Foods 2021, 10, 2629. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112629
Chen Y, Peng J, Wang Y, Wadhawan D, Wu L, Gao X, Sun Y, Xia G. Development, Characterization, Stability and Bioaccessibility Improvement of 7,8-Dihydroxyflavone Loaded Zein/Sophorolipid/Polysaccharide Ternary Nanoparticles: Comparison of Sodium Alginate and Sodium Carboxymethyl Cellulose. Foods. 2021; 10(11):2629. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112629
Chicago/Turabian StyleChen, Yufeng, Jingchong Peng, Yueqi Wang, Daniel Wadhawan, Lijun Wu, Xiaojing Gao, Yi Sun, and Guobin Xia. 2021. "Development, Characterization, Stability and Bioaccessibility Improvement of 7,8-Dihydroxyflavone Loaded Zein/Sophorolipid/Polysaccharide Ternary Nanoparticles: Comparison of Sodium Alginate and Sodium Carboxymethyl Cellulose" Foods 10, no. 11: 2629. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10112629