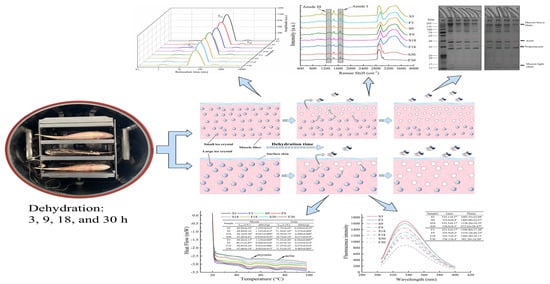
Exploring the Effect of Dehydration on Water Migrating Property and Protein Changes of Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Processing
2.2. Measurement of Weight Loss and Water Content
2.3. Measurement of Unfrozen Water Content
2.4. Low-Field Nuclear Magnetic Resonance (LF-NMR) and Magnetic Resonance Imaging (MRI)
2.5. Total Sulfhydryl (SH) Content
2.6. Determination of MFP Aggregation
2.6.1. Surface Hydrophobicity
2.6.2. Particle Size
2.7. Protein Secondary Structure
2.8. Protein Tertiary Structure
2.9. Protein Thermal Stability Properties
2.10. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss and Water Content Analysis
3.2. Water Status Analysis
3.3. Protein Oxidation
3.4. Protein Secondary Structure
3.5. Protein Tertiary Structure
3.6. MFP Aggregation
3.6.1. Surface Hydrophobicity
3.6.2. Particle Size
3.7. Protein Thermal Stability
3.8. SDS-PAGE Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalvi-Isfahan, M.; Jha, P.K.; Tavakoli, J.; Daraei-Garmakhany, A.; Xanthakis, E.; Le-Bail, A. Review on identification, underlying mechanisms and evaluation of freezing damage. J. Food Eng. 2019, 255, 50–60. [Google Scholar] [CrossRef]
- Tan, M.; Wang, J.; Li, P.; Xie, J. Storage time prediction of glazed frozen squids during frozen storage at different temperatures based on neural network. Int. J. Food Prop. 2020, 23, 1663–1677. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Q.; Sun, D.-W. Measuring and controlling ice crystallization in frozen foods: A review of recent developments. Trends Food Sci. Technol. 2019, 90, 13–25. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Mulot, V.; Benkhelifa, H.; Pathier, D.; Ndoye, F.-T.; Flick, D. Measurement of food dehydration during freezing in mechanical and cryogenic freezing conditions. Int. J. Refrig. 2019, 103, 329–338. [Google Scholar] [CrossRef]
- Rao, W.L.; Wang, Z.Y.; Shen, Q.W.; Li, G.X.; Song, X.; Zhang, D.Q. LF-NMR to explore water migration and water-protein interaction of lamb meat being air-dried at 35 degrees C. Dry. Technol. 2018, 36, 366–373. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Xie, J. Effect of Glazing with Different Materials on the Quality of Tuna During Frozen Storage. Foods 2020, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- Hottot, A.; Vessot, S.; Andrieu, J. Freeze drying of pharmaceuticals in vials: Influence of freezing protocol and sample configuration on ice morphology and freeze-dried cake texture. Chem. Eng. Process. Process Intensif. 2007, 46, 666–674. [Google Scholar] [CrossRef]
- Jin, J.; Yurkow, E.J.; Adler, D.; Lee, T.-C. Improved freeze drying efficiency by ice nucleation proteins with ice morphology modification. Food Res. Int. 2018, 106, 90–97. [Google Scholar] [CrossRef]
- Tan, M.; Mei, J.; Xie, J. The Formation and Control of Ice Crystal and Its Impact on the Quality of Frozen Aquatic Products: A Review. Crystals 2021, 11, 68. [Google Scholar] [CrossRef]
- Kumar, P.K.; Rasco, B.A.; Tang, J.; Sablani, S.S. State/Phase Transitions, Ice Recrystallization, and Quality Changes in Frozen Foods Subjected to Temperature Fluctuations. Food Eng. Rev. 2020, 12, 421–451. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Song, X.; Wang, W.; Bhandari, B. Changes in unfrozen water content and dielectric properties during pulse vacuum osmotic dehydration to improve microwave freeze-drying characteristics of Chinese yam. J. Sci. Food Agric. 2019, 99, 6572–6581. [Google Scholar] [CrossRef]
- Tan, M.; Li, P.; Yu, W.; Wang, J.; Xie, J. Effects of Glazing with Preservatives on the Quality Changes of Squid during Frozen Storage. Appl. Sci. 2019, 9, 3847. [Google Scholar] [CrossRef] [Green Version]
- Lan, W.; Hu, X.; Sun, X.; Zhang, X.; Xie, J. Effect of the number of freeze-thaw cycles number on the quality of Pacific white shrimp (Litopenaeus vannamei): An emphasis on moisture migration and microstructure by LF-NMR and SEM. Aquac. Fish. 2020, 5, 193–200. [Google Scholar] [CrossRef]
- Nian, L.; Cao, A.; Cai, L.; Ji, H.; Liu, S. Effect of vacuum impregnation of red sea bream (Pagrosomus major) with herring AFP combined with CS@Fe3O4 nanoparticles during freeze-thaw cycles. Food Chem. 2019, 291, 139–148. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, X.; Zhang, Z.; Long, G.; Lyu, F.; Cai, Y.; Liu, J.; Ding, Y. Effects of Immersion Freezing on Ice Crystal Formation and the Protein Properties of Snakehead (Channa argus). Foods 2020, 9, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-Y.; Xie, J. Evaluation of water dynamics and protein changes in bigeye tuna (Thunnus obesus) during cold storage. LWT 2019, 108, 289–296. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.-L.; Chen, S.-J.; Zhang, X.-L.; Wei, W.-Y. Influence of trehalose and alginate oligosaccharides on ice crystal growth and recrystallization in whiteleg shrimp (Litopenaeus vannamei) during frozen storage with temperature fluctuations. Int. J. Refrig. 2019, 99, 176–185. [Google Scholar] [CrossRef]
- Geidobler, R.; Winter, G. Controlled ice nucleation in the field of freeze-drying: Fundamentals and technology review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, D.; Zhong, X.; Pei, Z.; Tian, Y.; Xiang, D.; Cao, J.; Shen, X.; Li, C. Quality and protein degradation of golden pompano (Trachinotus blochii) fillets during four drying methods. LWT 2020, 130, 109638. [Google Scholar] [CrossRef]
- Cheng, S.; Tang, Y.; Zhang, T.; Song, Y.; Wang, X.; Wang, H.; Wang, H.; Tan, M. Approach for monitoring the dynamic states of water in shrimp during drying process with LF-NMR and MRI. Dry. Technol. 2018, 36, 841–848. [Google Scholar] [CrossRef]
- Song, Y.; Zang, X.; Kamal, T.; Bi, J.; Cong, S.; Zhu, B.; Tan, M. Real-time detection of water dynamics in abalone (Haliotis discus hannai Ino) during drying and rehydration processes assessed by LF-NMR and MRI. Dry. Technol. 2018, 36, 72–83. [Google Scholar] [CrossRef]
- Cai, L.; Cao, M.; Cao, A.; Regenstein, J.; Li, J.; Guan, R. Ultrasound or microwave vacuum thawing of red seabream (Pagrus major) fillets. Ultrason. Sonochemistry 2018, 47, 122–132. [Google Scholar] [CrossRef]
- Cai, L.; Nian, L.; Cao, A.; Zhang, Y.; Li, X. Effect of Carboxymethyl Chitosan Magnetic Nanoparticles Plus Herring Antifreeze Protein on Conformation and Oxidation of Myofibrillar Protein From Red Sea Bream (Pagrosomus major) After Freeze-Thaw Treatment. Food Bioprocess Technol. 2020, 13, 355–366. [Google Scholar] [CrossRef]
- Wang, B.; Kong, B.; Li, F.; Liu, Q.; Zhang, H.; Xia, X. Changes in the thermal stability and structure of protein from porcine longissimus dorsi induced by different thawing methods. Food Chem. 2020, 316, 126375. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Comparative study on physicochemical changes of muscle proteins from some tropical fish during frozen storage. Food Res. Int. 2003, 36, 787–795. [Google Scholar] [CrossRef]
- Gao, W.; Huang, Y.; Zeng, X.-A.; Brennan, M.A. Effect of soluble soybean polysaccharides on freeze-denaturation and structure of myofibrillar protein of bighead carp surimi with liquid nitrogen freezing. Int. J. Biol. Macromol. 2019, 135, 839–844. [Google Scholar] [CrossRef]
- Shao, J.-H.; Zou, Y.-F.; Xu, X.-L.; Wu, J.-Q.; Zhou, G.-H. Evaluation of structural changes in raw and heated meat batters prepared with different lipids using Raman spectroscopy. Food Res. Int. 2011, 44, 2955–2961. [Google Scholar] [CrossRef]
- Xiong, G.; Han, M.; Kang, Z.; Zhao, Y.; Xu, X.; Zhu, Y. Evaluation of protein structural changes and water mobility in chicken liver paste batters prepared with plant oil substituting pork back-fat combined with pre-emulsification. Food Chem. 2016, 196, 388–395. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, W.; Cao, A.; Cao, M.; Li, J. Effects of ultrasonics combined with far infrared or microwave thawing on protein denaturation and moisture migration of Sciaenops ocellatus (Red drum). Ultrason. Sonochemistry 2019, 55, 96–104. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, Q.; Xia, X.; Kong, B.; Diao, X. Effects of ultrasound-assisted freezing at different power levels on the structure and thermal stability of common carp (Cyprinus carpio) proteins. Ultrason. Sonochemistry 2019, 54, 311–320. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, C.-D.; Hao, G.-J.; Zhang, Y.-Y. Effect of kappa-carrageenan oligosaccharides on myofibrillar protein oxidation in peeled shrimp (Litopenaeus vannamei) during long-term frozen storage. Food Chem. 2018, 245, 254–261. [Google Scholar] [CrossRef]
- Zou, Y.; Xu, P.; Wu, H.; Zhang, M.; Sun, Z.; Sun, C.; Wang, D.; Cao, J.; Xu, W. Effects of different ultrasound power on physicochemical property and functional performance of chicken actomyosin. Int. J. Biol. Macromol. 2018, 113, 640–647. [Google Scholar] [CrossRef]
- Higuera-Barraza, O.A.; Torres-Arreola, W.; Ezquerra-Brauer, J.M.; Cinco-Moroyoqui, F.J.; Rodríguez Figueroa, J.C.; Marquez-Ríos, E. Effect of pulsed ultrasound on the physicochemical characteristics and emulsifying properties of squid (Dosidicus gigas) mantle proteins. Ultrason. Sonochemistry 2017, 38, 829–834. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.; Xia, X.; Kong, B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef]
- Saeed, S.; Howell, N.K. Rheological and differential scanning calorimetry studies on structural and textural changes in frozen Atlantic mackerel (Scomber scombrus). J. Sci. Food Agric. 2004, 84, 1216–1222. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Bu, Y.; Li, J.; Li, X. Effects of nanowarming on water holding capacity, oxidation and protein conformation changes in jumbo squid (Dosidicus gigas) mantles. LWT 2020, 129, 109511. [Google Scholar] [CrossRef]





| Sample | Bound Water | Immobilized Water | Free Water | UFW (g/100g) | Total SH Content (μmol/g) | |||
|---|---|---|---|---|---|---|---|---|
| T2b(ms) | A2b(1/g) | T21(ms) | A21(1/g) | T22(ms) | A22(1/g) | |||
| S3 | 1.96 ± 0.13 a | 257.11 ± 7.98 g | 54.79 ± 2.53 a | 21,341.01 ± 78.38 a | 622.26 ± 74.66 a | 313.22 ± 16.36 ab | 31.62 ± 0.78 a | 18.15 ± 0.26 a |
| S9 | 1.96 ± 0.12 a | 343.46 ± 4.86 f | 51.11 ± 0.49 b | 19,166.92 ± 67.84 b | 541.59 ± 57.91 b | 293.20 ± 9.10 c | 28.46 ± 0.52 c | 18.27 ± 0.23 a |
| S18 | 1.48 ± 0.12 bc | 385.73 ± 6.19 cd | 47.69 ± 1.28 c | 15,224.69 ± 59.93 ef | 439.76 ± 30.80 d | 231.89 ± 7.47 e | 23.99 ± 0.33 e | 17.98 ± 0.21 ab |
| S30 | 1.12 ± 0.14 cd | 459.91 ± 4.50 a | 41.50 ± 0.80 d | 11,908.50 ± 61.07 h | 410.27 ± 52.63 e | 164.98 ± 11.98 g | 19.10 ± 0.63 g | 16.84 ± 0.17 a |
| F3 | 1.96 ± 0.10 a | 246.29 ± 2.71 h | 54.79 ± 2.44 a | 19,077.90 ± 62.31 bc | 580.52 ± 61.45 ab | 337.16 ± 17.29 a | 29.08 ± 0.46 b | 18.22 ± 0.25 a |
| F9 | 1.70 ± 0.21 ab | 371.81 ± 4.74 e | 51.11 ± 0.99 b | 17,113.04 ± 65.26 d | 505.26 ± 28.38 c | 279.88 ± 13.14 cd | 28.53 ± 0.29 bc | 18.03 ± 0.20 a |
| F18 | 1.59 ± 0.12 b | 394.41 ± 4.33 c | 51.11 ± 1.56 ab | 16,077.90 ± 83.30 e | 471.38 ± 81.33 cd | 263.37 ± 20.26 d | 26.75 ± 0.57 d | 17.81 ± 0.24 b |
| F30 | 1.29 ± 0.11 c | 428.12 ± 5.18 b | 47.69 ± 3.27 c | 12,327.77 ± 107.5 g | 439.76 ± 101.26 d | 220.42 ± 28.53 ef | 21.04 ± 0.70 f | 17.05 ± 0.33 c |
| Sample | Myosin | Actin | ||
|---|---|---|---|---|
| Tmax1 (°C) | ∆H1 (J/g) | Tmax2 (°C) | ∆H2 (J/g) | |
| CK | 54.04 ± 0.45 a | 1.611 ± 0.01 a | 74.52 ± 0.30 a | 0.506 ± 0.014 b |
| S3 | 49.94 ± 0.25 b | 1.275 ± 0.012 b | 71.75 ± 0.23 b | 0.550 ± 0.012 ab |
| S9 | 48.80 ± 0.18 d | 1.122 ± 0.012 c | 71.10 ± 0.18 bc | 0.572 ± 0.004 a |
| S18 | 48.10 ± 0.30 e | 0.947 ± 0.008 e | 70.59 ± 0.75 c | 0.498 ± 0.006 b |
| S30 | 47.26 ± 0.41 f | 0.834 ± 0.014 gh | 70.72 ± 0.16 c | 0.517 ± 0.012 b |
| F3 | 49.42 ± 0.13 c | 1.129 ± 0.009 c | 71.35 ± 0.20 b | 0.564 ± 0.008 ab |
| F9 | 48.31 ± 0.49 de | 0.968 ± 0.010 d | 71.47 ± 0.16 b | 0.535 ± 0.014 b |
| F18 | 48.05 ± 0.24 e | 0.923 ± 0.006 f | 71.23 ± 0.42 b | 0.472 ± 0.010 c |
| F30 | 47.68 ± 0.19 f | 0.855 ± 0.013 e | 71.51 ± 0.35 b | 0.485 ± 0.003 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.; Xie, J. Exploring the Effect of Dehydration on Water Migrating Property and Protein Changes of Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage. Foods 2021, 10, 784. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040784
Tan M, Xie J. Exploring the Effect of Dehydration on Water Migrating Property and Protein Changes of Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage. Foods. 2021; 10(4):784. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040784
Chicago/Turabian StyleTan, Mingtang, and Jing Xie. 2021. "Exploring the Effect of Dehydration on Water Migrating Property and Protein Changes of Large Yellow Croaker (Pseudosciaena crocea) during Frozen Storage" Foods 10, no. 4: 784. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10040784