The individual phenolics present in
P. avium leaves, stems, and flowers were tentatively identified based on a comparison of their retention times, ultraviolet–visible spectra, and the interpretation of their fragmentation patterns as obtained from MS
n spectra and by their relationship with other data reported in the literature. This study identified a total of fifty-two phenolic compounds, some of which were previously described in
P. avium fruit [
4,
5,
7] and its by-products [
2,
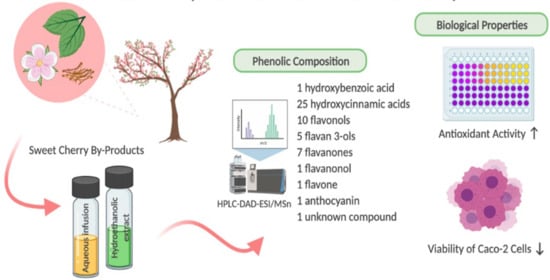
9]. The analysis allowed the identification of one hydroxybenzoic acid, twenty-five hydroxycinnamic acids, ten flavonols, five flavan-3-ols, seven flavanones, one flavanonol, one flavone, one anthocyanin, and one unknown compound (
Table 4 and
Table 5). As far as we know, this is the first study that characterizes the phenolic composition of leaves and flowers of sweet cherry, and the first concerned with the characterization of Saco variety stems from the Fundão region (Portugal), as evaluated by HPLC-DAD-ESI/MS
n.
3.3.2. Hydroxycinnamic Acids
A total of twenty-five hydroxycinnamic acids (peaks 1–4, 6–10, 12, 14–17, 20–21, 28, 33, 35, 38–39, 42, 44, 46–47) were identified in
P. avium by-products (
Table 4 and
Table 5) using previously developed studies [
2,
6,
9,
25,
26].
Peaks 1, 3, and 20 were identified as ferulic acids since they showed a fragmentation pattern at m/z 193 [ferulic acid-H]- after the loss of a hexosyl moiety (−162 mu), and the presence of a feruloyl fragment [
25]. Bastos et al. [
2] also detected their presence in
P. avium stem extracts, infusions, and decoctions. On the other hand, these compounds have also been described in sweet cherry fruits [
5,
6]. Feruloyl di-hexose (peak 1) was not quantified in all infusions and hydroethanolic extracts (
Table 5). Feruloyl hexose (peak 3) and feruloylquinic acid (peak 20) were not detected in leaves and stems, and it was not possible to quantify them in the flower infusion and hydroethanolic extract (
Table 5).
Twelve signals (peaks 2, 4, 6–7, 9–10, 15, 21, 33, 35, 38, and 39) were detected that eluted between 13.7 and 31.3 min (
Table 4). These components were identified as caffeoylquinic acids, and they have a fragmentation pattern at m/z 191 and/or at 179 [
27]. Caffeoylquinic acid-glycoside (peak 7), 3-caffeoylquinic acid
cis (peak 9), 3,5-dicaffeoylquinic acid 1 and 2 (peaks 10 and 35, respectively), 3,4-dicaffeoylquinic acid (peak 33), 5-caffeoylquinic acid
trans (peak 21), and 4,5-dicaffeoyquinic acid (peak 39) had already been reported by Martini et al. [
6] in sweet cherry cultivars. Moreover, peaks 4 and 7 were also identified as caffeoylquinic acid glycosides (m/z 515) (
Table 4 and
Table 5), and both possess a caffeoyl glucoside moiety (m/z 341) [
28]. Peak 17 corresponds to 4-caffeyolquinic acid (molecular ion m/z z 353) with a fragment pattern at m/z 173 [
25]. As far as we know, this is the first report concerning the presence of 4-caffeyolquinic acid in
P. avium leaf infusion (ca. 908.93 ± 91.95 µg/g of dw) and hydroethanolic extract (ca. 466.37 ± 19.7 µg/g of dw), as shown in
Table 5. However, its presence has already been reported in sweet cherries [
5,
6,
27].
Peaks 14 and 16 were identified as
p-coumaroylquinic acid derivative and
p-coumaric acid derivative, respectively. They exhibited a molecular ion at m/z 337 and fragmentation ions at m/z 191 (
Table 4 and
Table 5) [
2,
25,
28].
P-Coumaroylquinic acid has already been described in
P. avium stems [
2]. Moreover, peak 16 was described as a
p-coumaroylquinic acid derivative, showing fragmentation ions at m/z 337.
The phenolic compounds corresponding to the peaks 46 and 47 were classified as coumaroyl-caffeoylquinic acids (molecular ion at m/z 499) (
Table 4 and
Table 5). Nevertheless, they possess distinct fragment patterns due to dehydration and the loss of caffeoyl and
p-coumaroyl residues [
29]. 3-Coumaroyl-5-caffeoylquinic acid (peak 46) was identified only in sweet cherry fruits [
6].
Peak 12 was identified as a caffeoyl hexose (m/z 341) with a fragmentation at m/z 179 (
Table 4 and
Table 5) [
6]. Similarly, in another study, Bastos et al. [
2] tentatively identified these compounds as caffeic acid and trans-caffeic acid hexoside, based on the same pseudo-molecular ion, ultraviolet spectra, and fragmentation pattern. Additionally, two caffeoyl hexose derivatives (m/z 341) were tentatively identified (peaks 38 and 42), based on similar fragmentation (
Table 4 and
Table 5).
In sum, regarding hydroxycinnamic acids,
P. avium leaves proved to be the by-products richest in these types of compounds. Totals of 51345.69 and 57605.22 µg/g of dw were obtained for the infusion and hydroethanolic extract, respectively (
Table 5). Trans-5-caffeoylquinic acid (peak 21) was the major hydroxycinnamic acid found in both extracts, followed by 3-caffeoylquinic acid cis (peak 9). Our results were similar to those obtained by Jesus et al. [
9], who also reported that hydroxycinnamics were the main compounds in leaves, representing 75.3% and 63.7% of total phenolic compounds in the infusion and hydroethanolic extract, respectively.
3.3.3. Flavonols
Ten flavonols were found in
P. avium leaves, stems, and flowers (
Table 4 and
Table 5), according to previous studies [
2,
5,
30]. In this study, four quercetins (peaks 25, 26, 32, and 49) were detected (molecular ion at m/z 789, 771, 625, 609, and 463), as was a quercetin ion at m/z 301 (
Table 4 and
Table 5). Quercetin 3-O-rutinoside (peak 49) was the only flavonol quantified in all infusions and hydroethanolic extracts of
P. avium leaves, stems, and flowers (
Table 5). The leaf infusion was the richest one in this flavonol (ca. 6175.93 ± 148.22 µg/g of dw, comprising about 17.08% of total phenolic compounds), followed by flower hydroethanolic extract (1823.94 ± 38.9 µg/g of dw, containing about 7.71% of total phenolic compounds) (
Table 5). These results agree with other recent work [
9] showing that quercetin 3-
O-rutinoside was the major flavonol found in leaf extracts. According to the literature, flavonols are the main phenolics found in leaves, and quercetin 3-
O-rutinoside and quercetin 3-
O-glucoside were also found in
P. avium stems [
2]. On the other hand, peak 26 was tentatively identified as quercetin O-rutinoside-O-hexoside. This compound exhibits a molecular ion at m/z 771, and typical fragmentations at m/z 609, 463, and 301 (
Table 4). The presence of this flavonol in
P. avium by-products agrees with other previous work (Bastos et al., 2015). Additionally, peak 26 was identified as quercetin di-hexoside and was only quantified in the flower hydroethanolic extract (
Table 4 and
Table 5). Quercetin 3-
O-hexoside (peak 32) was detected at some levels in the stem and flower infusions and hydroethanolic extracts (
Table 4 and
Table 5), comprising less than 6% of total phenolic compounds.
On the other hand, six kaempferols (peaks 23, 27, 30, 34, 36, and 40) were found, all of which displayed a kaempferol fragment at m/z 285 [
28]. Kaempferol
O-rutinoside-
O-hexoside (peak 23) and kaempferol 3-glucoside (peak 36) were found for the first time by Bastos et al. [
2] in
P. avium stems. Our study, as we far as known, detected them for the first time in flowers and leaves (
Table 4 and
Table 5). Kaempferol 3-
O-rutinoside (peak 34) was identified in leaf infusions (1298.58 ± 44.2 µg/g) (
Table 4 and
Table 5), its presence being identified in previous works [
9].
3.3.4. Flavan 3-ols
Five flavan 3-ols (peaks 11, 13, 18–19, and 22) (
Table 4 and
Table 5) already described in cherries and their by-products were identified [
2,
5,
6,
9]. Catechin hexoside (peak 11) was tentatively identified, exhibiting a molecular ion at m/z 451 and fragmentation ions at m/z 289 and 245 (
Table 4). This catechin was found in leaves and stems of
P. avium (
Table 5).
Furthermore, four procyanidins were identified in our samples (peaks 13, 18, 19, and 22). Two of these compounds were tentatively classified as type-B procyanidin dimers at m/z 577 (peaks 13 and 22) (
Table 4 and
Table 5). The presence of a procyanidin is supported by the fragment patterns at m/z 425 as well as water elimination at m/z 407 [
28]. Our team reported similar findings for sweet cherry composition, which comprised about 18.47% total non-colored phenolics [
5]. Procyanidin dimer B type 1 was found in different sweet cherries [
6], while procyanidin dimer B type 2 was quantified in infusion and hydroethanolic extracts of
P. avium stems (7149.5 ± 510.5 and 8810.67 ± 592.2 µg/g of dw, respectively) (
Table 4 and
Table 5). Procyanidin tetramer (peak 18; m/z 1153 molecular ion) and procyanidin trimer (peak 19; m/z 865 molecular ion) were also identified in
P. avium by-products. As far as we know, this is the first study assessing the presence of these compounds in the leaves and stems of sweet cherries.
3.3.5. Flavanones
A total of seven flavanones, eluted between 31.0 and 39.1 min, were identified in
P. avium by-products, including four sakuranetins (peaks 37, 47, 51 and 52), two naringenins (peaks 41 and 48), and one pinocembrin (peak 44) (
Table 4 and
Table 5). Two sakuranetin 5-
O-hexoside derivatives (peaks 37 and 52) were found for the first time in all cherry by-products studied in this work (
Table 4 and
Table 5). Both compounds have a fragmentation at m/z 285. Sakuranetin 5
-O-hexoside (peak 51) was also identified in the same sample, presenting an ion at m/z 447 and releasing a fragment ion at m/z 285. This compound was quantified in the aqueous infusion and hydroethanolic extract (ca. 265.89 ± 9.8 and 214.66 ± 10.6 µg/g of dw, respectively) of
P. avium leaves (
Table 4), and these results are in accord with other previous works [
2,
9]. In addition, sakuranetin
O-pentosyl-hexoside (peak 48) was tentatively identified based on the same fragmentation previously described (
Table 4 and
Table 5), and was found in stems. Previous data have already reported the presence of sakuranin in sweet cherries [
21,
31,
32].
Naringenin-7-
O-hexoside (peak 41) and naringenin hexoside (peak 48) presented a similar pseudo molecular ion at m/z 433 and 443 (
Table 4 and
Table 5), respectively. They release a fragmentation ion at m/z 271 (loss of hexose group) [
2,
9]. Naringenin 7-
O-hexoside was quantified in stems infusion and hydroethanolic extracts (ca.1482.67 ± 15.94 and 1940.77 ± 51.2 µg/g of dw, respectively) (
Table 5), comprising about 12% of the total phenolic compounds present in cherry stems. This compound was already described in
P. avium stems and leaves, ranging between 51.9 and 4036.2 µg/g of dw, agreeing with other previous works [
9]. Naringenin hexoside was also reported in sweet cherries [
2,
6]. The compound detected in peak 44 was tentatively identified as pinocembrin
O-pentosyl-hexoside at a molecular ion m/z 549, releasing fragments at m/z 255 and 234. According to the literature, these fragments might be related to pinocembrin, a phenolic compound described in wood from different
Prunus species [
33]. A study conducted by Bastos et al. [
2] reported this flavanone in
P. avium stems extracts.