Candida Oleophila Proliferated and Accelerated Accumulation of Suberin Poly Phenolic and Lignin at Wound Sites of Potato Tubers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tubers, Yeast, and Fungi
2.2. Tuber Wounding
2.3. C. oleophila Treatment and Wound Healing
2.4. Determination of C. oleophila Population Dynamics at Wound Sites
2.5. Measurement of Weight Loss and Disease Index
2.6. Observation of SPP and Lignin Accumulation at Wounded Sites of Tubers
2.7. Sampling
2.8. Determination of Enzymes Activity
2.9. Determination of Protein Content
2.10. Determination of SPP Monomer Contents
2.11. Determination of Total Phenolic, Flavonoids, and Lignin Contents
2.12. Total RNA Extraction and qRT-PCR Analysis
2.13. Determination of H2O2 Content
2.14. Statistical Analysis
3. Results
3.1. Population Dynamics of C. oleophila at Wounded Sites
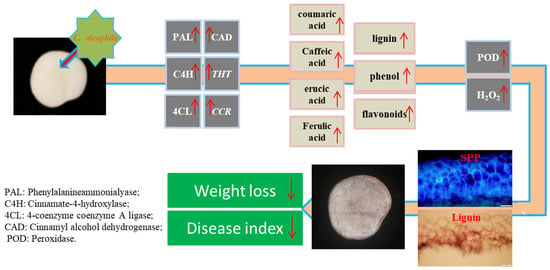
3.2. C. oleophila Reduced Weight Loss and Disease Index
3.3. C. oleophila Accelerated Accumulation of SPP and Lignin
3.4. C. oleophila Activated Key Enzyme of Phenylpropanoid Metabolism
3.5. C. oleophila Enhanced the Content of Phenolics and Lignin
3.6. C. oleophila Increased the H2O2 Content and POD Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernards, M.A. Demystifying suberin. Can. J. Bot. 2002, 80, 227–240. [Google Scholar] [CrossRef]
- Lulai, E.C. Skin-set, wound-healing and related defects. In Potato Biology and Biotechnology: Advances and Perspectives; Vreugdenhil, D., Ed.; Elsevier Limited: Amsterdam, The Netherlands, 2007; pp. 471–500. [Google Scholar] [CrossRef]
- Lulai, E.C.; Neubauer, J.D. Wound-induced suberization genes are differentially expressed, spatially and temporally, during closing layer and wound periderm formation. Postharvest Biol. Technol. 2014, 90, 24–33. [Google Scholar] [CrossRef]
- Yang, R.R.; Han, Y.; Han, Z.H.; Ackah, S.; Prusky, D. Hot water dipping stimulated wound healing of potato tubers. Postharvest Biol. Technol. 2020, 167, 111245. [Google Scholar] [CrossRef]
- Woolfson, K.N.; Haggitt, M.L.; Zhang, Y.; Kachura, A.; Bjelica, A.; Rincon, M.A.R.; Kaberi, K.M.; Bernards, M.A. Differential induction of polar and non-polar metabolism during wound-induced suberization in potato (Solanum tuberosum L.) tubers. Plant J. Cell Mol. Biol. 2018, 93, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Wang, B.; Ma, L.; Zheng, X.Y.; Gong, D.; Xue, H.L.; Bi, Y.; Wang, Y.; Zhang, Z.; Prusky, D. Benzo-(1, 2, 3)-thiadiazole-7-carbothioic acid s-methyl ester (BTH) promotes wound healing of potato tubers by eliciting the phenylpropanoid pathway. Postharvest Biol. Technol. 2019, 153, 125–132. [Google Scholar] [CrossRef]
- Carlos, T.U.; Prieto, V.G.; Cesar, G.L.; Francisco, V.A.; David, B.R.; Carlos, A.M.; Damaris, O.B. Purification and characterization of β-1, 3-glucanase from Candida Oleophila for the biocontrol of Penicillium expansum. J. Bot. Sci. 2016, 5, 38–45. [Google Scholar]
- Lahlali, R.; Serrhini, M.N.; Jijakli, M.H. Efficacy assessment of Candida oleophila (strain O) and Pichia anomala (strain K) against major postharvest diseases of citrus fruits in Morocco. Commun. Agric. Appl. Biol. Sci. 2004, 69, 601–609. [Google Scholar] [PubMed]
- Droby, S.; Wisniewski, M.E.; Ghaouth, A.; Wilson, C. Influence of food additives on the control of postharvest rots of apple and peach and efficacy of the yeast based biocontrol product Aspire. Postharvest Biol. Technol. 2003, 27, 127–135. [Google Scholar] [CrossRef]
- Gamagae, S.U.; Sivakumar, D.; Wijesundera, R.L.C. Evaluation of post-harvest application of sodium bicarbonate-incorporated wax formulation and Candida oleophila for the control of anthracnose of papaya. Crop Protention 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Elneshawy, S.M.; Wilson, C.L. Nisin enhancement of biocontrol of postharvest diseases of apple with Candida oleophila. Postharvest Biol. Technol. 1997, 10, 9–14. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Piombo, E.; Wu, X.H.; Yue, J.Y. Genome sequence, assembly, and characterization of the antagonistic yeast Candida oleophila used as a biocontrol agent against post-harvest diseases. Front. Microbiol. 2020, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droby, S.; Vinokur, V.; Weiss, B.; Cohen, L.; Daus, A.; Goldschmidt, E.E.; Porat, R. Induction of resistance to Penicillium digitatum in grape fruit by the yeast biocontrol agent Candida oleophila. Phytopathology 2002, 92, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wisniewski, M.; Artlip, T.; Sui, Y.; Droby, S.; Norelli, J. The potential role of PR-8 gene of apple fruit in the mode of action of the yeast antagonist, candida oleophila, in postharvest biocontrol of botrytis cinerea. Postharvest Biol. Technol. 2013, 85, 203–209. [Google Scholar] [CrossRef]
- Cai, M.X.; Zhou, Y.H.; Zhang, H.Y.; Deng, L.L.; Yao, S.X.; Zeng, K.F. Effect and possible modes of action of Candida oleophila on controlling Penicillium expansum in apples. Food Sci. 2018, 39, 265–271. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zong, Y.Y.; Li, Z.C.; Yang, R.R.; Li, Z.H.; Bi, Y.; Prusky, D. Postharvest Pichia guilliermondii treatment promotes wound healing of apple fruits. Postharvest Biol. Technol. 2020, 167, 111228. [Google Scholar] [CrossRef]
- Lulai, E.C.; Morgan, W.C. Histochemical probing of potato periderm with neutral red: A sensitive cytofluorochrome for the hydrophobic domain of suberin. Biotech. Histochem. 1992, 67, 185–195. [Google Scholar] [CrossRef]
- Oirschot, Q.E.A.V.; Rees, D.; Aked, J.; Kihurani, A. Sweetpotato cultivars differ in efficiency of wound healing. Postharvest Biol. Technol. 2006, 42, 65–74. [Google Scholar] [CrossRef]
- Kozukue, N.; Kozukue, E.; Kishiguchi, M. Changes in the contents of phenolic substances, phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL) accompanying chilling-injury of eggplant fruit. Sci. Hortic. 1979, 11, 51–59. [Google Scholar] [CrossRef]
- Lamb, C.J.; Rubery, P.H. A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity. Anal. Biochem. 1975, 68, 554–561. [Google Scholar] [CrossRef]
- Voo, K.S.; Whetten, R.W.; O’Malley, D.M.; Sederoff, R.R. 4-coumarate:coenzyme a ligase from loblolly pine xylem (isolation, characterization, and complementary DNA cloning). Plant Physiol. 1995, 108, 85–97. [Google Scholar] [CrossRef] [Green Version]
- Goffner, D.; Joffroy, I.; Grima-Pettenati, J.; Halpin, C.; Knight, M.E.; Schuch, W.; Boudet, A.M. Purification and characterization of isoforms of cinnamyl alcohol denydrogenase from Eucalyptus xylem. Planta 1992, 188, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Venisse, J.S.; Malnoy, M.; Faize, M.; Paulin, J.P.; Brisset, M.N. Modulation of defense responses of malus spp. during compatible and incompatible interactions with Erwinia amylovora. Mol. Plant Microbe. Interact. 2002, 15, 1204–1212. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Gruz, J.; Novank, O.; Strnad, M. Separation, characterization and quantitation of phenolic acids in a little-known blueberry (Vaccinium arctostaphylos L.) fruit by HPLC-MS. J. Agric. Food Chem. 2005, 53, 8116–8122. [Google Scholar] [CrossRef]
- Valcarcel, J.; Reilly, K.; Gaffney, M.; Brien, N.M.O. Antioxidant activity, total phenolic and total flavonoid content in sixty varieties of potato (solanum tuberosum) grown in ireland. Potato Res. 2015, 58, 221–244. [Google Scholar] [CrossRef]
- Morrison, I.M. A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage crops. J. Sci. Food Agric. 1972, 23, 455–463. [Google Scholar] [CrossRef]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Reference gene selection for rt-qpcrnormalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Bajji, M.; Mahmoud, M.H.; Gastiny, F.; Delaplace, P.; Fauconnier, M.L.; Jardin, P.D. Catalase inhibition alters suberization and wound healing in potato (Solanum tuberosum) tubers. Physiol. Plant. 2007, 129, 472–483. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, J.; Wisniewski, M.; Droby, S.; Norelli, J.; Hershkovitz, V. Pretreatment of the yeast antagonist, Candida oleophila, with glycine betaine increases oxidative stress tolerance in the microenvironment of apple wounds. Int. J. Food Microbiol. 2012, 157, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Andre, F.; Andre, R.C.; Flavio, T.S. Polymerization of lignin fragments contained in a model effluent by polyphenoloxidases and horseradish peroxidase/hydrogen peroxide system. Enzym. Microb. Technol. 2000, 26, 315–323. [Google Scholar] [CrossRef]
- Shadel, G.L.; Wesley, S.V.; Korth, K.L.; Cehn, F.; Lamb, C.; Dixon, R.A. Phenylpropanoid compounds and disease resistance in transgenic tobacco with altered expression of L-phenylalanine ammonia-lyase. Phytochemistry 2003, 64, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Scervino, J.M.; María, A.P.; Erra-Bassells, R.; Vierheilig, H.; Godeas, A. Flavonoids exhibit fungal species and genus specific effects on the presymbiotic growth of gigaspora and glomus. Mycol. Res. 2005, 109, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Morandi, D. Occurrence of phytoalexins and phenolic compounds in endomycorrhizal interactions, and their potential role in biological control. Plant Soil 1996, 185, 241–251. [Google Scholar] [CrossRef]
- Sun, C.; Fu, D.; Jin, L.F.; Chen, M.Y.; Zheng, X.D.; Yu, T. Chitin isolated from yeast cell wall induces the resistance of tomato fruit to Botrytis cinerea. Carbohydr. Polym. 2018, 199, 341–352. [Google Scholar] [CrossRef]
- Sun, C.; Jin, L.F.; Cai, Y.T.; Zheng, X.D.; Yu, T. (1→3)-β-D-glucan from yeast cell wall: Characteristic and potential application in controlling postharvest disease of pear. Postharvest Biol. Technol. 2019, 154, 105–114. [Google Scholar] [CrossRef]
- Ramamurthy, M.S.; Ussuf, K.K.; Nair, P.M.; Thomas, P. Lignin biosynthesis during wound healing of potato tubers in response to gamma irradiation. Postharvest Biol. Technol. 2000, 18, 267–272. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Iyer, S.; Knowles, N.R. Strboh A homologue of NADPH oxidase regulates wound-induced oxidative burst and facilitates wound-healing in potato tubers. Planta 2007, 227, 25–36. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ohura, I.; Kawakita, K.; Yokata, N.; Fujiwara, M.; Shimamoto, K.; Doke, N.; Yoshioka, H. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 2007, 19, 1065–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Berrocal-Lobo, M.; Stone, S.; Yang, X.; Antico, J.; Callis, J.; Ramonell, K.M.; Somerville, S. ATL9, a RING zinc finger protein with E3 ubiquitin ligase activity implicated in chitin and NADPH oxidase-mediated defense responses. PLoS ONE 2010, 5, e14426. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Jiang, H.; Silvy, E.M.; Zhao, S.; Chai, X.; Wang, B.; Li, Z.; Bi, Y.; Prusky, D. Candida Oleophila Proliferated and Accelerated Accumulation of Suberin Poly Phenolic and Lignin at Wound Sites of Potato Tubers. Foods 2021, 10, 1286. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10061286
Zheng X, Jiang H, Silvy EM, Zhao S, Chai X, Wang B, Li Z, Bi Y, Prusky D. Candida Oleophila Proliferated and Accelerated Accumulation of Suberin Poly Phenolic and Lignin at Wound Sites of Potato Tubers. Foods. 2021; 10(6):1286. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10061286
Chicago/Turabian StyleZheng, Xiaoyuan, Hong Jiang, Esrat Mahmud Silvy, Shijia Zhao, Xiuwei Chai, Bin Wang, Zhicheng Li, Yang Bi, and Dov Prusky. 2021. "Candida Oleophila Proliferated and Accelerated Accumulation of Suberin Poly Phenolic and Lignin at Wound Sites of Potato Tubers" Foods 10, no. 6: 1286. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10061286