Pomegranate Peel Powder as a Food Preservative in Fruit Salad: A Sustainable Approach
Abstract
:1. Introduction
2. Materials and Methods
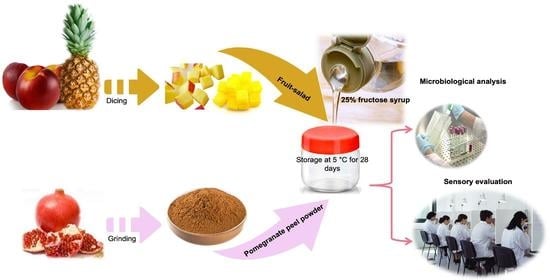
2.1. Raw Materials and Pomegranate Peel Powder
2.2. Fruit Salad Preparation
2.3. Microbiological Analysis
2.4. pH Determination
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microbial Quality Decay during Refrigerated Storage
3.2. Sensory Quality Decay during Refrigerated Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruiz Rodríguez, L.G.; Gasga, V.M.Z.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Aguayo, E.; Kader, A.A. Quality changes and nutrient retention in fresh-cut versus whole fruits during storage. J. Agric. Food Chem. 2006, 54, 4284–4296. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, E.; O’Beirne, D. Characterising deterioration patterns in fresh-cut fruit using principal component analysis. II: Effects of ripeness stage, seasonality, processing and packaging. Postharvest Biol. Technol. 2015, 100, 91–98. [Google Scholar] [CrossRef]
- Finnegan, E.; O’Beirne, D. Characterising and tracking deterioration patterns of fresh-cut fruit using principal component analysis—Part I. Postharvest Biol. Technol. 2015, 100, 73–80. [Google Scholar] [CrossRef]
- Li, X.; Long, Q.; Gao, F.; Jin, P.; Zheng, Y. Effect of cutting styles on quality and antioxidant activity in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Liu, S.; Liu, C.; Zheng, L. Effects of melatonin on the enzymatic browning and nutritional quality of fresh-cut pear fruit. Food Chem. 2019, 299, 125116. [Google Scholar] [CrossRef]
- Cortellino, G.; Gobbi, S.; Bianchi, G.; Rizzolo, A. Modified atmosphere packaging for shelf life extension of fresh-cut apples. Trends Food Sci. Technol. 2015, 46, 320–330. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Meireles, A.C.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016, 82, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Combined use of modified atmosphere packaging and natural compounds for food preservation. Food Eng. Rev. 2010, 2, 28–38. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Review: Sustainable use of fruit and vegetable by-products to enhance food packaging performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Pérez-Àlvarez, J.A.; Viuda-Martos, M. Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Akhatar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Review: Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Adnan, A.; Rizvi, S.S. Enzyme-assisted supercritical fluid extraction of phenolic antioxidants from pomegranate peel. J. Supercrit. Fluids 2015, 104, 122–131. [Google Scholar] [CrossRef]
- Molva, Ç.; Baysal, A.H. Evaluation of bioactivity of pomegranate fruit extract against Alicyclobacillus acidoterrestris DSM 3922 vegetative cells and spores in apple juice. LWT Food Sci. Technol. 2015, 62, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Nicosia, M.G.L.D.; Pangallo, S.; Raphael, G.; Romeo, F.V.; Strano, M.C.; Rapisarda, P.; Schena, L. Control of postharvest fungal rots on citrus fruit and sweet cherries using a pomegranate peel extract. Postharvest Biol. Technol. 2016, 114, 54–61. [Google Scholar] [CrossRef]
- Kharchoufi, S.; Licciardello, F.; Siracusa, L.; Muratore, G.; Hamdi, M.; Restuccia, C. Antimicrobial and antioxidant features of ‘Gabsi’ pomegranate peel extracts. Ind. Crops Prod. 2018, 111, 345–352. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Review: Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Huang, S.; Shi, Y.; Shao, Y.; Qiu, J.; Sedjoah, R.-C.A.-A.; Yan, Z.; Ding, L.; Zou, D.; Xin, Z. Extraction, isolation, characterization and antimicrobial activities of non-extractable polyphenols from pomegranate peel. Food Chem. 2021, 351, 129232. [Google Scholar] [CrossRef]
- Mushtaq, M.; Gani, A.; Gani, A.; Punoo, H.A.; Masoodi, F.A. Use of pomegranate peel extract incorporated zein film with improved properties for prolonged shelf life of fresh Himalayan cheese (Kalari/kradi). Innov. Food Sci. Emerg. Technol. 2018, 48, 25–32. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate-based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Gull, A.; Hat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef] [PubMed]
- Hanani, Z.A.N.; Husna, A.B.A.; Syahida, S.N.; Nor-Khaizura, M.A.B.; Jamilah, B. Effect of different fruit peels on the functional properties of gelatin/polyethylene bilayer films for active packaging. Food Packag. Shelf Life 2018, 18, 201–211. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef]
- Incoronato, A.L.; Cedola, A.; Conte, A.; Del Nobile, M.A. Juice and by-products from pomegranate to enrich pancake: Characterization and shelf-life evaluation. Int. J. Food Sci. Technol. 2021, 56, 2886–2894. [Google Scholar] [CrossRef]
- Panza, O.; Conte, A.; Del Nobile, M.A. Pomegranate by-products as natural preservative to prolong the shelf life of breaded cod stick. Molecules 2021, 26, 2385. [Google Scholar] [CrossRef] [PubMed]
- Majerska, J.; Michalska, A.; Figiel, A. A review of new directions in managing fruit and vegetable processing by-products. Trends Food Sci. Technol. 2019, 88, 207–219. [Google Scholar] [CrossRef]
- Costa, C.; Conte, A.; Buonocore, G.G.; Del Nobile, M.A. Antimicrobial silver montmorillonite nanoparticles to prolong the shelf life of fresh fruit salad. Int. J. Food Microbiol. 2011, 148, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Ministere de l’Economie des Finances et du Budget. Marché consommation, Produits vegetaux prets a l’emploi dits de la ‘IVemme Gamme’: Guide de bonnes pratique hygieniques. J. Officiel de la Republique Francaise 1988, 1621, 1–29. [Google Scholar]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and grape by-products and their active compounds: Are they a valuable source for food applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Yousuf, B.; Deshi, V.; Ozturk, B.; Siddiqui, M.W. Fresh-Cut Fruits and Vegetables: Quality Issues and Safety Concerns; Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–15. [Google Scholar]





| Samples | Storage Time (Days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 8 | 11 | 18 | 21 | 23 | 25 | 28 | ||
| Appearance | Control | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.5 ± 0.0 a | 4.5 ± 0.0 b | 3.5 ± 0.5 a | 3.4 ± 0.3 b | 3.3 ± 0.3 a | 3.4 ± 0.3 b | 2.9 ± 0.3 a |
| 2.5% | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.5 ± 0.5 a | 3.9 ± 0.3 a | 3.2 ± 0.3 a | 3.0 ± 0.0 a | 3.0 ± 0.0 a | 3.0 ± 0.0 a,b | 2.9 ± 0.3 a | |
| 5% | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.2 ± 0.3 a | 3.9 ± 0.3 a | 3.2 ± 0.3 a | 3.0 ± 0.0 a | 3.0 ± 0.0 a | 2.9 ± 0.3 a | 3.0 ± 0.0 a | |
| Odor | Control | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.5 ± 0.0 a | 4.0 ± 0.0 a | 3.8 ± 0.3 a | 3.2 ± 0.3 a | 2.8 ± 0.3 a | 2.9 ± 0.3 a | 2.3 ± 0.5 a |
| 2.5% | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 b | 4.5 ± 0.0 b | 3.9 ± 0.3 a | 2.9 ± 0.3 a | 2.8 ± 0.3 a | 3.0 ± 0.0 a | 3.0 ± 0.3 a | |
| 5% | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.5 ± 0.0 a | 4.5 ± 0.0 b | 3.5 ± 0.0 a | 2.9 ± 0.3 a | 2.9 ± 0.3 a | 2.9 ± 0.3 a | 2.7 ± 0.3 a | |
| Texture | Control | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.5 ± 0.0 a | 4.5 ± 0.0 a | 3.5 ± 0.0 a | 3.3 ± 0.3 a | 3.5 ± 0.0 a | 2.9 ± 0.3 a | 2.9 ± 0.3 a |
| 2.5% | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.5 ± 0.0 a | 4.5 ± 0.0 a | 3.9 ± 0.3 a | 3.5 ± 0.0 a | 3.3 ± 0.3 a | 3.3 ± 0.3 a | 3.0 ± 0.3 a | |
| 5% | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 5.0 ± 0.0 a | 4.5 ± 0.0 a | 4.5 ± 0.0 a | 3.9 ± 0.3 a | 3.5 ± 0.0 a | 3.4 ± 0.3 a | 3.2 ± 0.3 a | 3.0 ± 0.0 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacivita, V.; Incoronato, A.L.; Conte, A.; Del Nobile, M.A. Pomegranate Peel Powder as a Food Preservative in Fruit Salad: A Sustainable Approach. Foods 2021, 10, 1359. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10061359
Lacivita V, Incoronato AL, Conte A, Del Nobile MA. Pomegranate Peel Powder as a Food Preservative in Fruit Salad: A Sustainable Approach. Foods. 2021; 10(6):1359. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10061359
Chicago/Turabian StyleLacivita, Valentina, Anna Lucia Incoronato, Amalia Conte, and Matteo Alessandro Del Nobile. 2021. "Pomegranate Peel Powder as a Food Preservative in Fruit Salad: A Sustainable Approach" Foods 10, no. 6: 1359. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10061359