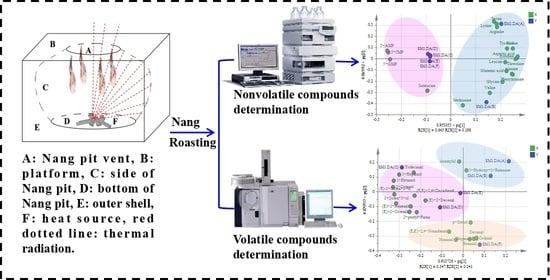
Comprehensive Evaluation of Volatile and Nonvolatile Compounds in Oyster Cuts of Roasted Lamb at Different Processing Stages Using Traditional Nang Roasting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Materials
2.3. Sample Preparation
2.4. Proximate Analysis
2.5. Volatile Compounds Analysis
2.5.1. HS-SPME Analysis of the Volatile Compounds
2.5.2. Qualitative and Quantitative Analysis of the Volatile Compounds
2.5.3. Odor Activity Value (OAV) of the Volatile Compounds
2.6. Determination of Nonvolatile Compounds
2.6.1. Determination of Free Amino Acid
2.6.2. Determination of Nucleotides
2.6.3. Taste Activity Value (TAV) of the Nonvolatile Compounds
2.6.4. Equivalent Umami Concentration (EUC) of Nonvolatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes of Proximate Compositions in Nang Roasted Oyster Cuts at Different Processing Stages
3.2. Changes of Volatile Compounds in Nang Roasted Oyster Cuts at Different Processing Stages
3.2.1. The Profiles of Volatile Compounds
3.2.2. The Key Volatile Compounds
3.2.3. Changes of Key Volatiles Compounds in Nang Roasted Oyster Cuts at Different Processing Stages
3.3. Changes of Nonvolatile Compounds in Nang Roasted Oyster Cuts at Different Processing Stages
3.3.1. The Profiles of Free Amino Acids
3.3.2. The Key Free Amino Acids
3.3.3. The Profiles of 5′-Nucleotides
3.3.4. The Key 5′-Nucleotides
3.3.5. Changes of the Key Nonvolatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Roldan, M.; Ruiz, J.; del Pulgar, J.S.; Pérez-Palacios, T.; Antequera, T. Volatile compound profile of sous-vide cooked lamb loins at different temperature–time combinations. Meat Sci. 2015, 100, 52–57. [Google Scholar] [CrossRef]
- Xiao, X.; Hou, C.; Zhang, D.; Li, X.; Ren, C.; Ijaz, M.; Hussain, Z.; Liu, D. Effect of pre- and post-rigor on texture, flavor, heterocyclic aromatic amines and sensory evaluation of roasted lamb. Meat Sci. 2020, 169, 108220. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, Y.; Jin, Y.; Deng, Y.; Zhao, Y. Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Petričević, S.; Radovčić, N.M.; Lukić, K.; Listeš, E.; Medić, H. Differentiation of dry-cured hams from different processing methods by means of volatile compounds, physico-chemical and sensory analysis. Meat Sci. 2018, 137, 217–227. [Google Scholar] [CrossRef]
- Han, D.; Mi, S.; Zhang, C.-H.; Li, J.; Song, H.-L.; Fauconnier, M.-L.; Tyteca, E. Characterization and Discrimination of Chinese Marinated Pork Hocks by Volatile Compound Profiling Using Solid Phase Microextraction Gas Chromatography-Mass Spectrometry/Olfactometry, Electronic Nose and Chemometrics. Molecules 2019, 24, 1385. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, C.; Chen, S. Comparison of Active Non-volatile Taste Components in the Viscera and Adductor Muscles of Oyster (Ostrea rivularis Gould). Food Sci. Technol. Res. 2013, 19, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ma, J.; Pan, T.; Suleman, R.; Wang, Z.; Zhang, D. Effects of roasting by charcoal, electric, microwave and superheated steam methods on (non)volatile compounds in oyster cuts of roasted lamb. Meat Sci. 2021, 172, 108324. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Xu, C.-H.; Chen, G.-S.; Xiong, Z.-H.; Fan, Y.-X.; Wang, X.-C.; Liu, Y. Applications of solid-phase microextraction in food analysis. TrAC Trends Anal. Chem. 2016, 80, 12–29. [Google Scholar] [CrossRef]
- Xi, J.; Zhan, P.; Tian, H.; Wang, P. Effect of Spices on the Formation of VOCs in Roasted Mutton Based on GC-MS and Principal Component Analysis. J. Food Qual. 2019, 2019, 8568920. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, R.; Yang, X.; Gao, Z.; Yuan, Y.; Yue, T. Changes in aroma components and potential Maillard reaction products during the stir-frying of pork slices. Food Control. 2021, 123, 107855. [Google Scholar] [CrossRef]
- Wall, K.R.; Kerth, C.R.; Miller, R.K.; Alvarado, C. Grilling temperature effects on tenderness, juiciness, flavor and volatile aroma compounds of aged ribeye, strip loin, and top sirloin steaks. Meat Sci. 2019, 150, 141–148. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Wang, Y.; Kong, B.; Chen, Q. Evaluation of the flavour properties of cooked chicken drumsticks as affected by sugar smoking times using an electronic nose, electronic tongue, and HS-SPME/GC-MS. LWT 2021, 140, 110764. [Google Scholar] [CrossRef]
- Zou, Y.; Kang, D.; Liu, R.; Qi, J.; Zhou, G.; Zhang, W. Effects of ultrasonic assisted cooking on the chemical profiles of taste and flavor of spiced beef. Ultrason. Sonochem. 2018, 46, 36–45. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.-H.; Fauconnier, M.-L.; Jia, W.; Wang, J.-F.; Hu, F.-F.; Xie, D.-W. Characterization and comparison of flavor compounds in stewed pork with different processing methods. LWT 2021, 144, 111229. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburgh, MD, USA, 2000. [Google Scholar]
- Liu, P.; Wang, S.; Zhang, H.; Wang, H.; Kong, B. Influence of glycated nitrosohaemoglobin prepared from porcine blood cell on physicochemical properties, microbial growth and flavour formation of Harbin dry sausages. Meat Sci. 2019, 148, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Hui, T.; Ma, J. Generation of key aroma compounds in Beijing roasted duck induced via Maillard reaction and lipid pyrolysis reaction. Food Res. Int. 2020, 136, 109328. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.-L.; Zhou, G.-H. Changes in taste compounds of duck during processing. Food Chem. 2007, 102, 22–26. [Google Scholar] [CrossRef]
- Qi, J.; Liu, D.; Zhou, G.; Xu, X. Characteristic Flavor of Traditional Soup Made by Stewing Chinese Yellow-Feather Chickens. J. Food Sci. 2017, 82, 2031–2040. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshikawa, T.; Ikeda, S.; Ninomiya, T. Measurement of the relative taste intensity of some l-?-amino acids and 5′-nucleotides. J. Food Sci. 1971, 36, 846–849. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Morton, J.; Clerens, S.; Dyer, J. Cooking-Induced Protein Modifications in Meat. Compr. Rev. Food Sci. Food Saf. 2017, 16, 141–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisan, A.J.B.; Lima, D.D.A.; Sampaio, G.R.; Soares, R.A.M.; Bastos, D.H.M. Influence of home cooking conditions on Maillard reaction products in beef. Food Chem. 2016, 196, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Hu, M.; Wang, S.; Liu, Q.; Cao, R.; Xue, Y. Flavor profile of dried shrimp at different processing stages. LWT 2021, 146, 111403. [Google Scholar] [CrossRef]
- Watanabe, A.; Kamada, G.; Imanari, M.; Shiba, N.; Yonai, M.; Muramoto, T. Effect of aging on volatile compounds in cooked beef. Meat Sci. 2015, 107, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Ya-Ting, J.; Jin-Xuan, C.; Yin-Ji, C.; Yang-Ying, S.; Xiao-Qun, Z.; Dao-Dong, P.; Chang-Rong, O.; Ning, G. Study on lipolysis-oxidation and volatile flavour compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar] [CrossRef]
- Ma, Q.; Hamid, N.; Oey, I.; Kantono, K.; Faridnia, F.; Yoo, M.; Farouk, M. Effect of chilled and freezing pre-treatments prior to pulsed electric field processing on volatile profile and sensory attributes of cooked lamb meats. Innov. Food Sci. Emerg. Technol. 2016, 37, 359–374. [Google Scholar] [CrossRef]
- Machiels, D. Gas chromatography-olfactometry analysis of beef meat originating from differently fed Belgian Blue, Limousin and Aberdeen Angus bulls. Food Chem. 2004, 86, 377–383. [Google Scholar] [CrossRef]
- Salum, P.; Guclu, G.; Selli, S. Comparative Evaluation of Key Aroma-Active Compounds in Raw and Cooked Red Mullet (Mullus barbatus) by Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2017, 65, 8402–8408. [Google Scholar] [CrossRef]
- Benet, I.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J.; Roura, E. Analysis of SPME or SBSE extracted volatile compounds from cooked cured pork ham differing in intramuscular fat profiles. LWT 2015, 60, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, X.; Zhang, C.-H.; Wang, J.-Z.; Tang, C.-H.; Chen, L.-L. Flavor compounds and sensory profiles of a novel Chinese marinated chicken. J. Sci. Food Agric. 2015, 96, 1618–1626. [Google Scholar] [CrossRef]
- Kawai, M.; Okiyama, A.; Ueda, Y. Taste Enhancements between Various Amino Acids and IMP. Chem. Senses 2002, 27, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.-Y.; Tao, N.-P.; Gong, J.; Gu, S.-Q.; Xu, C.-H. Comparison of non-volatile taste-active compounds between the cooked meats of pre- and post-spawning Yangtze Coilia ectenes. Fish. Sci. 2015, 81, 559–568. [Google Scholar] [CrossRef]
- Chen, D.-W.; Zhang, M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis). Food Chem. 2007, 104, 1200–1205. [Google Scholar] [CrossRef]
- Watanabe, A.; Tsuneishi, E.; Takimoto, Y. Analysis of ATP and Its Breakdown Products in Beef by Reversed-Phase HPLC. J. Food Sci. 1989, 54, 1169–1172. [Google Scholar] [CrossRef]
- Mateo, J.; Domínguez, M.C.; Aguirrezábal, M.M.; Zumalacárregui, J. Taste compounds in chorizo and their changes during ripening. Meat Sci. 1996, 44, 245–254. [Google Scholar] [CrossRef]


| Physicochemical Properties | Nang Roasting Stages | |||||
|---|---|---|---|---|---|---|
| Fresh Oyster Cuts | Marinated Oyster Cuts | Roasted-15 | Roasted-30 | Roasted-45 | Roasted-60 | |
| Moisture/% | 72.90 ± 2.89 a | 73.03 ± 1.31 a | 67.46 ± 1.52 b | 64.27 ± 1.14 c | 60.36 ± 1.71 d | 59.05 ± 1.28 d |
| Protein/% | 69.95 ± 1.33 b | 65.31 ± 0.61 d | 71.94 ± 2.22 a | 72.06 ± 1.52 a | 67.35 ± 1.09 cd | 69.65 ± 2.31 b |
| Fat/% | 18.12 ± 0.64 a | 18.08 ± 0.55 a | 15.44 ± 1.52 b | 15.09 ± 0.94 b | 14.94 ± 1.46 c | 10.41 ± 0.48 d |
| Compounds | Lri a | Identification d | Ions (m/z) | Nang Roasting Stages | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Literature b | Calculated c | Fresh Oyster Cuts | Marinated Oyster Cuts | Roasted-15 | Roasted-30 | Roasted-45 | Roasted-60 | |||
| Aldehydes | - | - | - | - | - | - | - | - | - | - |
| Hexanal | 1074 | 1077 | MS + LRI | 44 | ND | 512.81 ± 62.59 c | 367.48 ± 161.23 c | 3025.93 ± 163.3 b | 3250.66 ± 269.46 b | 5728.83 ± 1202.9 a |
| Heptanal | 1174 | 1178 | MS + LRI | 70 | ND | 161.81 ± 8.43 b | ND | 522.17 ± 87.42 a | ND | 435.07 ± 107.89 a |
| Octanal | 1277 | 1280 | MS + LRI | 41 | ND | ND | ND | ND | ND | 853.20 ± 249.03 |
| Nonanal | 1385 | 1384 | MS + LRI | 57 | ND | 711.44 ± 112.43 c | 676.48 ± 65.64 c | 2275.44 ± 371.36 a | 1104.52 ± 234.80 b | 2392.88 ± 727.74 a |
| Decanal | 1483 | 1493 | MS + LRI | 41 | ND | ND | ND | ND | ND | 75.9 ± 13.51 a |
| Tridecanal | 1824 | 1812 | MS + LRI | 57 | ND | ND | 104.65 ± 38.26 a | ND | ND | ND |
| 2-Undecenal | 1755 | 1746 | MS + LRI | 70 | ND | ND | ND | ND | 120.13 ± 0.43 a | ND |
| Tetradecanal | 1931 | 1920 | MS + LRI | 57 | ND | ND | ND | ND | ND | 29.78 ± 7.34 a |
| Hexadecanal | 2141 | 2134 | MS + LRI | 57 | ND | ND | ND | ND | ND | 35.57 ± 6.41 a |
| Heptadecanal | 2249 | 2247 | MS + LRI | 82 | ND | ND | ND | 21.95 ± 12.24 a | ND | ND |
| (E)-2-Octenal | 1416 | 1419 | MS + LRI | 41 | ND | 98.57 ± 0.86 c | 272.52 ± 47.24 a | 247.39 ± 83.16 a | 174.91 ± 15.74 a b | 151.60 ± 61.84 b |
| (E)-2-Nonenal | 1535 | 1525 | MS + LRI | 41 | ND | 67.37 ± 1.41 c | 282.33 ± 71.79 a | 172.58 ± 75.42 b | 177.36 ± 51.07 b | 133.14 ± 14.17 b |
| (E)-2-Decenal | 1634 | 1636 | MS + LRI | 43 | ND | ND | 142.60 ± 9.20 a | 79.75 ± 14.65 b | ND | 49.57 ± 17.23 b |
| Benzaldehyde | 1508 | 1507 | MS + LRI | 77 | ND | 16.45 ± 2.97 a | ND | ND | ND | ND |
| (E, E)-2,4-Nonadienal | 1686 | 1692 | MS + LRI | 81 | ND | ND | ND | 65.89 ± 15.84 a | 50.19 ± 4.74 b | 49.78 ± 7.08 b |
| (E, E)-2,4-Decadienal | 1804 | 1801 | MS + LRI | 81 | ND | ND | 261.21 ± 35.65 a | 105.12 ± 19.17 b | 62.73 ± 14.06 c | 69.81 ± 16.66 c |
| Alcohols | - | - | - | - | - | - | - | - | - | - |
| isoamylol | 1210 | 1217 | MS + LRI | 55 | 254.57 ± 36.22 c | 756.99 ± 175.33 a | 436.68 ± 69.39 a b | ND | ND | ND |
| 1-Pentanol | 1252 | 1251 | MS + LRI | 42 | 15.20 ± 8.22 c | 58.10 ± 15.49 c | 479.89 ± 176.27 a | 348.63 ± 66.75 b a | 222.77 ± 17.65 b | 169.02 ± 30.61 b c |
| 1-Hexanol | 1358 | 1353 | MS + LRI | 56 | 64.70 ± 5.44 d | 204.91 ± 57.69 d | 3130.19 ± 1363.53 a | 2524.30 ± 569.71 b c | 1616.83 ± 293.23 c | ND |
| 1-Heptanol | 1456 | 1455 | MS + LRI | 70 | ND | 152.66 ± 19.93 c | 476.22 ± 137.41 a | 263.20 ± 95.07 b | 325.37 ± 67.09 a b | ND |
| 1-Octanol | 1554 | 1557 | MS + LRI | 56 | 21.86 ± 0.53 d | 80.97 ± 14.61 d | 781.59 ± 284.57 a | 372.65 ± 153.69 b | 367.63 ± 155.73 b | 194.47 ± 63.05 c |
| 1-Nonanol | 1661 | 1662 | MS + LRI | 55 | ND | ND | 496.34 ± 186.77 a | ND | 302.89 ± 101.73 b | ND |
| 1-Octen-3-ol | 1451 | 1450 | MS + LRI | 57 | 112.03 ± 6.43e | 535.24 ± 157.33 d | 1689.58 ± 632.75 a | 1394.20 ± 199.32 a b | 876.69 ± 62.15 b c | 915.46 ± 235.34 b c |
| Benzyl alcohol | 1866 | 1868 | MS + LRI | 79 | 2.00 ± 0.87 b | ND | 12.76 ± 5.76 a | ND | ND | ND |
| Phenylethyl alcohol | 1909 | 1906 | MS + LRI | 91 | 17.54 ± 8.39 a | 24.75 ± 8.17 a | ND | ND | ND | ND |
| Eucalyptol | 1204 | 1212 | MS + LRI | 43 | ND | ND | 172.43 ± 42.48 a | 49.39 ± 11.86 b | 36.08 ± 11.23 b | ND |
| 2,3-Butanediol | 1570 | 1573 | MS + LRI | 45 | 82.69 ± 0.28 d | 208.04 ± 9.46 c | 561.34 ± 74.25 a | 494.15 ± 88.12 b | 453.92 ± 234.05 b | ND |
| (E)-2-Octen-1-ol | 1617 | 1611 | MS + LRI | 57 | ND | 106.30 ± 8.83 d | 463.70 ± 129.76 a | 272.49 ± 42.86 b | 171.70 ± 8.02 c | 166.11 ± 54.00 c |
| (E)-2-Nonen-1-ol | 1722 | 1713 | MS + LRI | 57 | ND | ND | 32.47 ± 18.48 a | ND | ND | ND |
| Terpinen-4-ol | 1595 | 1598 | MS + LRI | 71 | ND | 78.33 ± 23.51 a | 85.51 ± 16.62 a | ND | ND | ND |
| alpha. -Terpineol | 1688 | 1695 | MS + LRI | 59 | ND | 58.97 ± 21.35 a | ND | ND | ND | ND |
| Ketones | - | - | - | - | - | - | - | - | - | - |
| 3-Hydroxy-2-Butanone | 1275 | 1278 | MS + LRI | 45 | 1050.67 ± 205.78 c | 1121.36 ± 294.36 c | 1283.36 ± 103.87 b | 1235.51 ± 98.14 b | 2597.47 ± 207.43 a | ND |
| 3-Octen-2-one | 1414 | 1410 | MS + LRI | 55 | ND | ND | ND | 36.57 ± 11.66 b | ND | 70.74 ± 25.62 a |
| 2,3-Octanedione | 1325 | 1325 | MS + LRI | 43 | ND | 138.63 ± 8.40 d | 501.72 ± 97.99 c | ND | 1244.92 ± 629.25 b | 2508.95 ± 804.81 a |
| 2-Undecanone | 1325 | 1325 | MS + LRI | 58 | ND | ND | 15.95 ± 5.46 a | ND | 13.47 ± 2.24 a | 10.45 ± 2.66 a |
| Esters | - | - | - | - | - | - | - | - | - | - |
| n-Caproic acid vinyl ester | - | - | MS | 43 | 37.10 ± 1.37 c | ND | 628.57 ± 67.99 a | 639.57 ± 108.82 a | 522.29 ± 142.11 b | 483.70 ± 19.01 b |
| Acids | - | - | - | - | - | - | - | - | - | - |
| Butanoic acid | 1663 | 1666 | MS + LRI | 60 | 54.31 ± 13.01 c | 108.43 ± 31.83 a b | 133.81 ± 2.36 a | 81.90 ± 16.41 b | 67.84 ± 12.36 c | ND |
| Hexanoic acid | 1849 | 1839 | MS + LRI | 60 | ND | 125.60 ± 23.46 c | 423.90 ± 84.10 a | 242.26 ± 46.37 b | 218.23 ± 30.96 b | 85.64 ± 20.42 c |
| Octanoic acid | 2060 | 2056 | MS + LRI | 60 | ND | 7.85 ± 2.56 a | ND | ND | ND | ND |
| Alkanes | - | - | - | - | - | - | - | - | - | - |
| 1-Pentadecene | 1545 | 1545 | MS + LRI | 41 | ND | ND | ND | ND | 21.84 ± 5.38 a | ND |
| Styrene | 1252 | 1250 | MS + LRI | 104 | ND | 29.05 ± 9.57 b | ND | 41.32 ± 7.66 ab | 52.30 ± 10.54 a | ND |
| Dodecane | - | - | MS | 57 | ND | ND | 84.23 ± 12.69 a | ND | ND | ND |
| Tetradecane | - | - | MS | 57 | ND | ND | 123.99 ± 24.92 a | ND | ND | ND |
| Heptadecane | - | - | MS | 57 | ND | ND | 87.10 ± 12.44 a | ND | 31.99 ± 7.09 b | 27.45 ± 11.11 b |
| Others | - | - | - | - | - | - | - | - | - | - |
| Phenol | 1992 | 1994 | MS + LRI | 94 | ND | ND | ND | ND | 163.16 ± 14.96 a | 136.06 ± 13.51 b |
| p-Cresol | 2068 | 2059 | MS + LRI | 107 | ND | ND | ND | ND | 62.02 ± 5.72 a | 65.02 ± 18.17 a |
| Anethole | 1818 | 1817 | MS + LRI | 148 | ND | ND | 39.14 ± 9.40 a | 15.86 ± 8.08 ab | 11.39 ± 1.60 b | ND |
| Biphenyl | 1981 | 1981 | MS + LRI | 154 | ND | ND | ND | 17.69 ± 7.22 b | 37.47 ± 3.40 a | ND |
| 2-pentyl-Furan | 1230 | 1216 | MS + LRI | 81 | ND | 55.03 ± 5.07 b | 138.53 ± 27.65 a | 91.33 ± 18.89 ab | 93.14 ± 36.30 ab | 109.95 ± 32.26 ab |
| methoxy-phenyl-Oxime | - | - | MS | 133 | 291.84 ± 53.06 c | 385.81 ± 115.95 b | 529.29 ± 164.69 a | 371.59 ± 52.09 b | 310.00 ± 69.05 b | 107.70 ± 24.25 cd |
| 1,2,3,4-tetramethyl-Benzene | 1430 | 1424 | MS + LRI | 119 | ND | 11.59 ± 5.09 a | 32.55 ± 17.56 a | ND | ND | ND |
| Free Amino Acid(mg/100 g) | Nang Roasting Stages | Taste Threshold (mg/100 g) | |||||
|---|---|---|---|---|---|---|---|
| Fresh Oyster Cuts | Marinated Oyster Cuts | Roasted-15 | Roasted-30 | Roasted-45 | Roasted-60 | ||
| Aspartic acid | 23.28 ± 2.61 a | 19.23 ± 1.02 a | 2.87 ± 0.57 b | 2.79 ± 0.52 b | 2.33 ± 0.59 b | 1.98 ± 0.29 b | 100 |
| Glutamic acid | 416.35 ± 11.99 b | 461.39 ± 11.22 a | 329.74 ± 13.71 b | 318.87 ± 12.57 bc | 282.82 ± 10.19 c | 264.89 ± 1.66 c | 30 |
| ∑UAA | 439.63 ± 9.61 b | 480.62 ± 9.12 a | 332.61 ± 12.57 c | 321.66 ± 10.27 c | 285.15 ± 5.19 d | 266.87 ± 4.62 d | / |
| Threonine | 22.42 ± 2.96 a | 19.99 ± 1.15 a | 7.39 ± 0.68 b | 6.92 ± 1.51 b | 6.78 ± 0.51 b | 5.46 ± 1.00 b | 260 |
| Serine | 19.98 ± 2.43 a | 5.17 ± 0.26 b | 5.72 ± 0.37 b | 6.41 ± 0.97 b | 5.96 ± 0.40 b | 5.34 ± 0.81 b | 150 |
| Proline | 190.23 ± 7.26 a | 115.81 ± 9.67 b | 41.30 ± 1.88 c | 27.99 ± 1.65 c d | 21.88 ± 11.05 d | 14.33 ± 1.92e | 300 |
| Glycine | 7.92 ± 0.57 b | 15.39 ± 1.66 a | 1.95 ± 0.39 c | 1.81 ± 0.72 c | 2.23 ± 0.93 c | 3.19 ± 0.39 c | 130 |
| Alanine | 84.16 ± 6.02 b | 100.94 ± 5.53 a | 52.74 ± 1.79 c | 40.46 ± 2.19 d | 39.79 ± 1.14 d | 30.60 ± 1.75 d | 60 |
| ∑SAA | 324.71 ± 10.56 a | 257.3 ± 6.57 b | 109.1 ± 3.83 c | 83.59 ± 4.57 d | 76.64 ± 2.43 d | 58.92 ± 5.35e | / |
| Valine | 4.74 ± 0.26 b | 16.47 ± 1.30 a | 2.02 ± 0.48 b | 1.82 ± 0.17 b | 2.00 ± 0.44 b | 1.99 ± 0.38 b | 40 |
| Methionine | 0.21 ± 0.05 d | 3.95 ± 0.19 a | 1.16 ± 0.46 c | 0.65 ± 0.20 d | 1.40 ± 0.03 bc | 1.89 ± 0.45 b | 30 |
| Leucine | 12.20 ± 2.51 a | 12.00 ± 1.72 a | 3.58 ± 0.82 b | 4.13 ± 0.93 b | 5.88 ± 0.76 b | 4.74 ± 0.37 b | 190 |
| Tyrosine | 30.04 ± 4.45 a | 19.86 ± 2.56 b | 5.59 ± 1.84 c | 8.88 ± 1.18 c | 10.23 ± 1.53 bc | 10.06 ± 1.32 bc | 91 |
| Isoleucine | 1.21 ± 0.62 c | 3.40 ± 0.03 a | 2.35 ± 0.98 b | 2.68 ± 0.50 b | 3.05 ± 0.91 a | 3.45 ± 1.22 a | 90 |
| Phenylalanine | 16.28 ± 2.37 a | 22.89 ± 1.28 a | ND | ND | ND | ND | 90 |
| Lysine | 22.36 ± 2.39 a | 3.17 ± 0.78 b | 4.72 ± 0.32 b | 4.37 ± 0.99 b | 5.89 ± 1.38 b | 4.44 ± 0.61 b | 50 |
| Arginine | 15.68 ± 1.14 a | 0.11 ± 0.03 b | ND | ND | ND | ND | 50 |
| Histidine | 13.89 ± 1.00 a | 10.30 ± 0.70 b | ND | ND | ND | ND | 20 |
| ∑BAA | 116.61 ± 3.92 a | 92.15 ± 3.28 b | 19.42 ± 1.42 c | 22.53 ± 1.95 c | 28.45 ± 2.36 c | 26.57 ± 1.87 c | / |
| Cystine | 14.16 ± 1.91 b | 28.21 ± 2.86 a | 6.54 ± 0.88 c | 5.97 ± 0.68 c | 6.89 ± 1.38 c | 7.50 ± 1.40 c | / |
| ∑OAA | 14.16 ± 1.91 b | 28.21 ± 2.86 a | 6.54 ± 0.88 c | 5.97 ± 0.68 c | 6.89 ± 1.38 c | 7.50 ± 1.40 c | / |
| ∑FAA | 704.88 ± 28.72 b | 858.30 ± 30.56 a | 467.67 ± 15.83 c | 433.75 ± 16.18 c | 397.14 ± 13.05 cd | 361.02 ± 14.32 d | / |
| Nucleotide (mg/100 g) | Nang Roasting Stages | Taste Threshold (mg/100 g) | |||||
|---|---|---|---|---|---|---|---|
| Fresh Oyster Cuts | Marinated Oyster Cuts | Roasted-15 | Roasted-30 | Roasted-45 | Roasted-60 | ||
| 5′-GMP | 0.39 ± 0.23 b | 0.82 ± 0.42 b | 2.82 ± 0.45 a | 1.90 ± 0.28 a | 2.35 ± 0.18 a | 2.30 ± 0.03 a | 12.50 |
| 5′-IMP | 6.65 ± 2.01 b | 4.35 ± 1.80 b | 98.35 ± 37.39 a | 76.23 ± 22.25 a | 96.08 ± 21.17 a | 97.56 ± 3.39 a | 25 |
| 5′-AMP | 5.38 ± 0.78 c | 4.69 ± 0.56 c | 23.64 ± 7.27 b | 31.97 ± 1.41 a | 34.34 ± 2.62 a | 30.85 ± 0.55 a | 50 |
| 5′-ADP | 61.09 ± 3.35 b | 69.86 ± 7.11 a | 6.19 ± 1.79 c | 5.02 ± 0.64 c | 6.02 ± 1.21 c | 7.49 ± 0.99 c | / |
| Hx | 40.72 ± 7.81 b c | 196.38 ± 48.86 a | 69.16 ± 14.55 b | 23.55 ± 3.15 c | 22.17 ± 4.95 c | 15.77 ± 2.03 c | / |
| I | 18.47 ± 4.82 c | 17.79 ± 1.88 c | 45.87 ± 3.03 c | 80.4 ± 13.89 b | 124.45 ± 7.35 a b | 161.88 ± 31.08 a | / |
| Flavor 5′-nucleotides | 12.42 ± 3.79 b | 9.86 ± 1.93 b | 124.81 ± 28.04 a | 110.10 ± 1.10 a | 132.77 ± 18.10 a | 130.71 ± 2.96 a | / |
| EUC (g MSG/100 g) | 4.76 ± 0.23 b | 4.45 ± 0.51 b | 44.17 ± 5.28 a | 33.88 ± 2.41 a | 37.39 ± 4.13 a | 35.26 ± 2.89 a | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhang, D.; Liu, H.; Wang, Z.; Hui, T.; Sun, J. Comprehensive Evaluation of Volatile and Nonvolatile Compounds in Oyster Cuts of Roasted Lamb at Different Processing Stages Using Traditional Nang Roasting. Foods 2021, 10, 1508. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10071508
Xu Y, Zhang D, Liu H, Wang Z, Hui T, Sun J. Comprehensive Evaluation of Volatile and Nonvolatile Compounds in Oyster Cuts of Roasted Lamb at Different Processing Stages Using Traditional Nang Roasting. Foods. 2021; 10(7):1508. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10071508
Chicago/Turabian StyleXu, Yujun, Dequan Zhang, Huan Liu, Zhenyu Wang, Teng Hui, and Jilu Sun. 2021. "Comprehensive Evaluation of Volatile and Nonvolatile Compounds in Oyster Cuts of Roasted Lamb at Different Processing Stages Using Traditional Nang Roasting" Foods 10, no. 7: 1508. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10071508