Evaluation of Anthocyanin Profiles in Various Blackcurrant Cultivars over a Three-Year Period Using a Fast HPLC-DAD Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Preparation of Solutions
2.3. Sample Preparation by Extraction
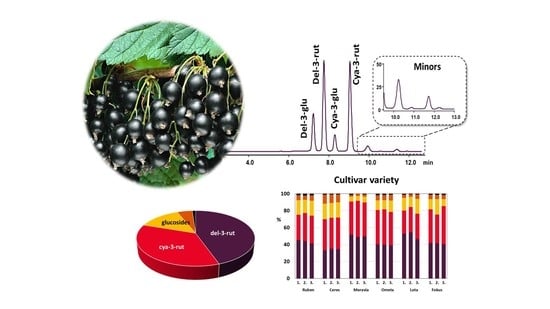
2.4. HPLC Analysis
3. Results and Discussion
3.1. HPLC Method Optimization
3.2. Method Validation
3.3. Evaluation of Individual and Total Anthocyanin Content in Blackcurrant Berries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mattila, P.H.; Hellström, J.; Karhu, S.; Pihlava, J.-M.; Veteläinen, M. High variability in flavonoid contents and composition between different north-european currant (Ribes spp.) varieties. Food Chem. 2016, 204, 14–20. [Google Scholar] [CrossRef]
- Frøytlog, C.; Slimestad, R.; Andersen, Ø.M. Combination of chromatographic techniques for the preparative isolation of anthocyanins—Applied on blackcurrant (ribes nigrum) fruits. J. Chrom. A 1998, 825, 89–95. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Technol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Ponder, A.; Hallmann, E.; Kwolek, M.; Średnicka-Tober, D.; Kazimierczak, R. Genetic differentiation in anthocyanin content among berry fruits. Curr. Issues Mol. Biol. 2021, 43, 4. [Google Scholar] [CrossRef]
- Bordonaba, J.G.; Crespo, P.; Terry, L.A. A new acetonitrile-free mobile phase for hplc-dad determination of individual anthocyanins in blackcurrant and strawberry fruits: A comparison and validation study. Food Chem. 2011, 129, 1265–1273. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Aaby, K.; Sønsteby, A.; Heide, O.M.; Wold, A.-B.; Remberg, S.F. Influence of controlled postflowering temperature and daylength on individual phenolic compounds in four black currant cultivars. J. Agric. Food Chem. 2016, 64, 752–761. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Slimestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agrict. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Karjalainen, R.O. High-performance liquid chromatography analysis of black currant (Ribes nigrum L.) fruit phenolics grown either conventionally or organically. J. Agric. Food Chem. 2006, 54, 7530–7538. [Google Scholar] [CrossRef] [PubMed]
- Šmídová, B.; Šatínský, D.; Dostálová, K.; Solich, P. The pentafluorophenyl stationary phase shows a unique separation efficiency for performing fast chromatography determination of highbush blueberry anthocyanins. Talanta 2017, 166, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Cacace, J.E.; Mazza, G. Extraction of anthocyanins and other phenolics from black currants with sulfured water. J. Agric. Food Chem. 2002, 50, 5939–5946. [Google Scholar] [CrossRef] [PubMed]
- Havlíková, L.; Míková, K. Heat stability of anthocyanins. Z. Für Lebensm.-Unters. Und Forsch. 1985, 181, 427–432. [Google Scholar] [CrossRef]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O.; de Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Jasutiene, I.; Venskutonis, P.R.; Viskelis, P. Hplc determination of the composition and stability of blackcurrant anthocyanins. J. Chromatogr. Sci. 2005, 43, 478–482. [Google Scholar] [CrossRef]
- Sáenz-López, R.; Fernández-Zurbano, P.; Tena, M.T. Development and validation of a capillary zone electrophoresis method for the quantitative determination of anthocyanins in wine. J. Chromatogr. A 2003, 990, 247–258. [Google Scholar] [CrossRef]
- Keith, E.S.; Powers, J.J. Gas chromatographic determination of anthocyanins and other flavonoids as silyl derivatives. J. Food Sci. 1966, 31, 971–979. [Google Scholar] [CrossRef]
- Baj, A.; Bombardelli, E.; Gabetta, B.; Martinelli, E.M. Qualitative and quantitative evaluation of vaccinium myrtillus anthocyanins by high-resolution gas chromatography and high-performance liquid chromatography. J. Chromatogr. A 1983, 279, 365–372. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. Ascorbic acid, anthocyanins, organic acids and mineral content of some black and red currant cultivars. Fruits 2011, 66, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Rennaker, C.; Wrolstad, R.E. Correlation of two anthocyanin quantification methods: Hplc and spectrophotometric methods. Food Chem. 2008, 110, 782–786. [Google Scholar] [CrossRef]
- Rubinskiene, M.; Vjakelis, P.; Jasutieo, I.; Duchovskis, P.; Bobinas, C. Changes in biologically active constituents during ripening in black currants. J. Fruit Ornam. Plant Res. 2006, 14, 237–246. [Google Scholar]
- Gibson, L.; Rupasinghe, H.P.V.; Forney, C.F.; Eaton, L. Characterization of changes in polyphenols, antioxidant capacity and physico-chemical parameters during lowbush blueberry fruit ripening. Antioxidants 2013, 2, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (Ribes nigrum L.): Variation due to genotype, location, and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef]
- Iversen, C.K. Black currant nectar: Effect of processing and storage on anthocyanin and ascorbic acid content. J. Food Sci. 1999, 64, 37–41. [Google Scholar] [CrossRef]
- Milivojevic, J.; Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Nikolic, M.; Veberic, R. The influence of early yield on the accumulation of major taste and health-related compounds in black and red currant cultivars (Ribes spp.). J. Agric. Food Chem. 2012, 60, 2682–2691. [Google Scholar] [CrossRef]
- Matejicek, A.; Kaplan, J.; Matejickova, J.; Kaplanova, M.; Smidova, B. Evaluation of blackcurrant cultivars for use as a table fruit. Acta Hortic. 2016, 1139, 265–268. [Google Scholar] [CrossRef]
- Scalzo, J.; Stevenson, D.; Hedderley, D. Blueberry estimated harvest from seven new cultivars: Fruit and anthocyanins. Food Chem. 2013, 139, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Bordonaba, J.G.; Terry, L.A. Biochemical profiling and chemometric analysis of seventeen uk-grown black currant cultivars. J. Agric.Food Chem. 2008, 56, 7422–7430. [Google Scholar] [CrossRef] [PubMed]
- Rachtan-Janicka, J.; Ponder, A.; Hallmann, E. The Effect of Organic and Conventional Cultivations on Antioxidants Content in Blackcurrant (Ribes nigrum L.) Species. Appl. Sci. 2021, 11, 5113. [Google Scholar] [CrossRef]




| Analyte | tR (min) | Peak Resolution | Peak Symmetry 1 | Capacity Factor Pc 2 | Precision (RSD, %) 3 | Recovery (%) 4 |
|---|---|---|---|---|---|---|
| Delphinidin-3-glucoside | 7.42 | - | 1.01 | 3.18 | 2.06 | 92.63 |
| Delphinidin-3-rutinoside | 7.92 | 2.09 | 1.04 | 3.50 | 1.81 | 90.10 |
| Cyanidin-3-glucoside | 8.56 | 2.06 | 1.13 | 3.81 | 1.40 | 91.07 |
| Cyanidin-3-rutinoside | 9.30 | 3.04 | 1.12 | 4.27 | 1.90 | 90.88 |
| Cultivars | Total Anthocyanins (Cya-3-Glu Equivalent) | Sum of 4 Major Anthocyanins | Del-3-Rut | Cya-3-Rut | Del-3-Glu | Cya-3-Glu |
|---|---|---|---|---|---|---|
| Ometa | 231.76 | 310.98 | 137.66 | 129.09 | 30.86 | 13.36 |
| Ceres | 212.15 | 261.82 | 97.08 | 101.71 | 42.22 | 20.82 |
| Lota | 171.55 | 219.95 | 125.92 | 60.73 | 28.60 | 4.70 |
| Ben Lomond | 169.58 | 217.38 | 120.32 | 73.68 | 18.95 | 4.42 |
| Ruben | 165.72 | 208.30 | 103.94 | 64.59 | 31.66 | 8.11 |
| Tenah | 150.67 | 202.02 | 84.49 | 78.57 | 29.13 | 9.83 |
| Sejanec | 169.21 | 186.27 | 74.88 | 72.29 | 26.46 | 12.63 |
| Consort | 127.19 | 172.56 | 89.49 | 58.28 | 20.06 | 4.74 |
| Fokus | 110.70 | 141.42 | 64.08 | 57.51 | 15.68 | 4.15 |
| Moravia | 103.66 | 135.78 | 74.30 | 53.10 | 7.80 | 0.58 |
| Ben Conan | 62.80 | 79.41 | 40.17 | 25.77 | 11.84 | 1.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimerdová, B.; Bobríková, M.; Lhotská, I.; Kaplan, J.; Křenová, A.; Šatínský, D. Evaluation of Anthocyanin Profiles in Various Blackcurrant Cultivars over a Three-Year Period Using a Fast HPLC-DAD Method. Foods 2021, 10, 1745. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10081745
Šimerdová B, Bobríková M, Lhotská I, Kaplan J, Křenová A, Šatínský D. Evaluation of Anthocyanin Profiles in Various Blackcurrant Cultivars over a Three-Year Period Using a Fast HPLC-DAD Method. Foods. 2021; 10(8):1745. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10081745
Chicago/Turabian StyleŠimerdová, Barbora, Michaela Bobríková, Ivona Lhotská, Jiří Kaplan, Alena Křenová, and Dalibor Šatínský. 2021. "Evaluation of Anthocyanin Profiles in Various Blackcurrant Cultivars over a Three-Year Period Using a Fast HPLC-DAD Method" Foods 10, no. 8: 1745. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10081745