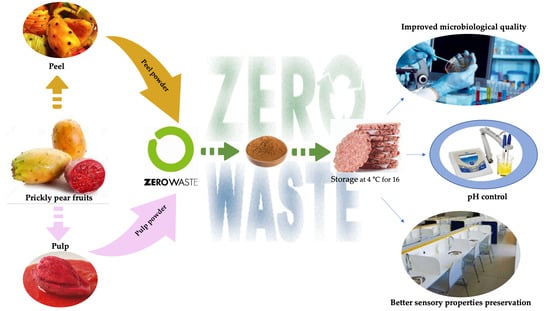
Improving the Storability of Cod Fish-Burgers According to the Zero-Waste Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prickly Pears
2.2. Fish Burger Formulation
2.3. Prickly Pear Powder Antibacterial Activity: In Vitro Test
2.4. Microbiological Analyses and pH Evaluation
2.5. Color Evaluation
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effects of Prickly Pear Powder on Pseudomonas spp. by In Vitro Test
3.2. Microbial Contamination of Prickly Pear Powder
3.3. Effects of Prickly Pear Powder on Microbiological Quality of Cod Fish Burgers
3.4. Effects of Prickly Pear Powder on pH of Cod Fish Burgers
3.5. Effects of Prickly Pear Powder on Sensory Quality of Cod Fish Burgers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aka, S.; Buyukdag, N. How to prevent food waste behaviour? A deep empirical research. J. Retail. Consum. Serv. 2021, 61, 102560. [Google Scholar] [CrossRef]
- Song, Q.; Li, J.; Zeng, X. Minimizing the increasing solid waste through zero waste strategy. J. Clean. Prod. 2015, 104, 199–210. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef]
- Karimi, A.; Kazemi, M.; Amiri Samani, S.; Simal-Gandara, J. Bioactive compounds from by-products of eggplant: Functional properties, potential applications and advances in valorization methods. Trends Food Sci. Technol. 2021, 112, 518–531. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Sendra, E.; Carbonell-Barrachina, Á.; Legua, P.; Hernández, F. Fatty acid profile of fruits (pulp and peel) and cladodes (young and old) of prickly pear [Opuntia ficus-indica (L.) Mill.] from six Spanish cultivars. J. Food Compos. Anal. 2019, 84, 103294. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredient in biscuits formulation. LWT Food Sci. Technol. 2020, 124, 109155. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Jimenez Martinez, C.; Sandoval, G. Revalorization of cactus pear (Opuntia spp.) wastes as a source of antioxidants. Food Sci. Technol. 2011, 31, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Palmeri, R.; Parafati, L.; Arena, E.; Grassenio, E.; Restuccia, C.; Fallico, B. Antioxidant and antimicrobial properties of semi-processed frozen prickly pear juice as affected by cultivar and harvest time. Foods 2020, 9, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabagias, V.K.; Karabagias, I.K.; Prodromiti, M.; Gatzias, I.; Badeka, A. Bio-functional alcoholic beverage preparation using prickly pear juice and its pulp in combination with sugar and blossom honey. Food Biosci. 2020, 35, 100591. [Google Scholar] [CrossRef]
- Valero-Galván, J.; González-Fernández, R.; Sigala-Hernández, A.; Núnez-Gastélum, J.A.; Ruiz-May, E.; Rodrigo-García, J.; Larqué-Saavedra, A.; del Rocío Martínez-Ruiz, N. Sensory attributes, physicochemical and antioxidant characteristics, and protein profile of wild prickly pear fruits (O. macrocentra Engelm., O. phaeacantha Engelm., and O. engelmannii Salm-Dyck ex Engelmann.) and commercial prickly pear fruits (O. ficus-indica (L.) Mill.). Food Res. Int. 2021, 140, 109909. [Google Scholar] [CrossRef]
- Palmeri, R.; Parafati, L.; Restuccia, C.; Fallico, B. Application of prickly pear fruit extract to improve domestic shelf life, quality and microbial safety of sliced beef. Food Chem. Toxicol. 2018, 118, 355–360. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crop. Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Restuccia, C.; Palmeri, R.; Fallico, B.; Arena, E. Characterization of prickly pear peel flour as a bioactive and functional ingredient in bread preparation. Foods 2020, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Danza, A.; Conte, A.; Del Nobile, M.A. Technological options to control quality of fish burgers. J. Food Sci. Technol. 2017, 54, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Conte, A.; Lecce, L.; Incoronato, A.L.; Del Nobile, M.A. Microencapsulated propolis to enhance the antioxidant properties of fresh fish burgers. J. Food Proc. Eng. 2014, 38, 527–535. [Google Scholar] [CrossRef]
- Parlapani, F.F. Microbial diversity of seafood. Curr. Opin. Food Sci. 2021, 37, 45–51. [Google Scholar] [CrossRef]
- Houicher, A.; Bensid, A.; Regenstein, J.M.; Ozogul, F. Control of biogenic amine production and bacterial growth in fish and seafood products using phytochemicals as biopreservatives: A review. Food Biosci. 2021, 39, 100807. [Google Scholar] [CrossRef]
- Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Innovative seafood preservation technologies: Recent developments. Animals 2021, 11, 92. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [Green Version]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M.; Hasani, M. Nano-encapsulation of lemon essential oil approach to reducing the oxidation process in fish burger during refrigerated storage. J. Food Biosci. Technol. 2020, 10, 35–46. [Google Scholar]
- Cedola, A.; Cardinali, A.; Del Nobile, M.A.; Conte, A. Fish burger enriched by olive oil industrial by-product. Food Sci. Nutr. 2017, 5, 837–844. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Corbo, M.R.; Speranza, B.; Sinigaglia, M.; Conte, A.; Caroprese, M. Combined effect of MAP and active compounds on fresh blue fish burger. Int. J. Food Microbiol. 2009, 135, 281–287. [Google Scholar] [CrossRef]
- Albertos, I.; Marrtin-Diana, A.B.; Burón, M.; Rico, D. Development of functional bio-based seaweed (Himanthalia elongata and Palmaria palmata) edible films for extending the shelf life of fresh fish burgers. Food Packag. Shelf Life 2019, 22, 100382. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Ennouri, M.; Ammar, I.; Khemakhem, B.; Attia, H. Chemical composition and antibacterial activity of Opuntia Ficus-Indica F. Inermis (Cactus Pear) Flowers. J. Med. Food 2014, 17, 908–914. [Google Scholar] [CrossRef]
- Rico, D.; Albertos, I.; Martinez-Alvarez, O.; Lopez-Caballero, M.E.; Martin-Diana, A.B. Use of sea fennel as a natural ingredient of edible films for extending the shelf life of fresh fish burgers. Molecules 2020, 25, 5260. [Google Scholar] [CrossRef]
- Panza, O.; Conte, A.; Del Nobile, M.A. Pomegranate by-products as natural preservative to prolong the shelf life of breaded cod stick. Molecules 2021, 26, 2385. [Google Scholar] [CrossRef]
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wang, Z.; Guo, Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin-based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019, 124, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Mexis, S.F.; Chouliara, E.; Kontominas, M.G. Combined effect of an oxygen absorber and oregano essential oil on shelf life extension of rainbow trout fillets stored at 4 °C. Food Microbiol. 2009, 26, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Feng, L.; Jiang, T.; Zhu, J.; Fu, L.; Yuan, D.; Li, J. The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Kitundu, E.; Young, O.; Seale, B.; Owens, A. Lactic fermentation of cooked, comminuted mussel, Perna canaliculus. Food Microbiol. 2021, 99, 103829. [Google Scholar] [CrossRef] [PubMed]
- Danza, A.; Lucera, A.; Lavermicocca, P.; Lonigro, S.L.; Bavaro, A.R.; Mentana, A.; Centonze, D.; Conte, A.; Del Nobile, M.A. Tuna Burgers Preserved by the Selected Lactobacillus paracasei IMPC 4.1 Strain. Food Bioprocess. Technol. 2018, 11, 1651–1661. [Google Scholar] [CrossRef]
- Kharrat, N.; Salem, H.; Mrabet, A.; Aloui, F.; Triki, S.; Fendri, A.; Gargouri, Y. Synergistic effect of polysaccharides, betalain pigment and phenolic compounds of red prickly pear (Opuntia stricta) in the stabilization of salami. Int. J. Biol. Macromol. 2018, 111, 561–568. [Google Scholar] [CrossRef]







| Ingredients | CNT | ACT-2.5 | ACT-7.5 | ACT-12.5 | ||||
|---|---|---|---|---|---|---|---|---|
| Weight [g] | Weight [%] | Weight [g] | Weight [%] | Weight [g] | Weight [%] | Weight [g] | Weight [%] | |
| Cod fillet | 36.98 | 73.9 | 36.98 | 70.4 | 36.98 | 64.3 | 36.98 | 59.1 |
| Extra-virgin olive oil | 4.62 | 9.2 | 4.62 | 8.8 | 4.62 | 8.0 | 4.62 | 7.4 |
| Potato starch | 4.62 | 9.2 | 4.62 | 8.8 | 4.62 | 8.0 | 4.62 | 7.4 |
| Potato flakes | 3.7 | 7.4 | 3.7 | 7.0 | 3.7 | 6.4 | 3.7 | 5.9 |
| Salt | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 |
| Prickly pear powder | - | - | 2.5 | 4.8 | 7.5 | 13.0 | 12.5 | 20.0 |
| log CFU/mL | |||||
|---|---|---|---|---|---|
| Sample Time (h) | 0 | 4 | 24 | 48 | 72 |
| Ctrl | 2.96 ± 0.11 | 3.46 ± 0.09 | 8.82 ± 0.39 | 9.41 ± 0.17 | 9.49 ± 0.23 |
| Peel 2.5% | 3.12 ± 0.01 | 3.62 ± 0.09 | 1.30 ± 0.43 | <10 | <10 |
| Peel 5% | 3.26 ± 0.08 | 4.05 ± 0.14 | 2.09 ± 0.12 | <10 | <10 |
| Pulp 2.5% | 3.57 ± 0.21 | 3.80 ± 0.14 | 1.35 ± 0.49 | <10 | <10 |
| Pulp 5% | 3.51 ± 0.01 | 3.54 ± 0.09 | 2.76 ± 0.09 | <10 | <10 |
| Sample | PSY | MES | PSE | ENTER | LAB | SHPB |
|---|---|---|---|---|---|---|
| Peel | - | 7.14 ± 0.20 | 3.43 ± 0.10 | 4.71 ± 0.11 | 7.07 ± 0.10 | 6.02 ± 0.53 |
| Pulp | - | 7.03 ± 0.04 | 3.56 ± 0.04 | 3.35 ± 0.16 | 7.12 ± 0.16 | 5.15 ± 0.27 |
| Samples | Storage Time (day) | ||||
|---|---|---|---|---|---|
| Raw Fish Burger | Cooked Fish Burger | ||||
| 0 | 16 | 0 | 16 | ||
| Color | CNT | 8.1 ± 0.25 b | 2.9 ± 0.25 c | 8.3 ± 0.29 b | 4.1 ± 0.25 b |
| ACT-2.5 | 9.0 ± 0.10 a | 4.5 ± 0.58 b | 9.0 ± 0.10 a | 4.8 ± 0.29 b | |
| ACT-7.5 | 9.0 ± 0.10 a | 6.1 ± 0.48 a | 9.0 ± 0.10 a | 6.3 ± 0.87 a | |
| ACT-12.5 | 9.0 ± 0.10 a | 6.4 ± 0.75 a | 9.0 ± 0.10 a | 7.0 ± 0.41 a | |
| Odor | CNT | 9.0 ± 0.10 a | 2.4 ± 0.25 d | 9.0 ± 0.10 a | 3.5 ± 0.58 b |
| ACT-2.5 | 9.0 ± 0.10 a | 3.6 ± 0.48 c | 9.0 ± 0.10 a | 3.6 ± 0.25 b | |
| ACT-7.5 | 9.0 ± 0.10 a | 6.0 ± 0.29 b | 9.0 ± 0.10 a | 6.4 ± 0.48 a | |
| ACT-12.5 | 9.0 ± 0.10 a | 6.9 ± 0.25 a | 9.0 ± 0.10 a | 7.0 ± 0.41 a | |
| Texture | CNT | 8.1 ± 0.25 b | 3.6 ± 0.25 c | 8.3 ± 0.29 a | 4.0 ± 0.41 c |
| ACT-2.5 | 8.9 ± 0.25 a | 5.8 ± 0.50 b | 8.8 ± 0.29 a | 4.8 ± 0.29 b | |
| ACT-7.5 | 9.0 ± 0.10 a | 6.5 ± 0.41 a,b | 8.9 ± 0.25 a | 7.0 ± 0.41 a | |
| ACT-12.5 | 8.9 ± 0.25 a | 7.0 ± 0.71 a | 8.6 ± 0.48 a | 7.3 ± 0.29 a | |
| Samples | L* | a* | b* | |||
|---|---|---|---|---|---|---|
| 0 | 16 | 0 | 16 | 0 | 16 | |
| CNT | 67.73 ± 1.87 a,A | 68.68 ± 1.97 a,A | −2.79 ± 0.81 b,A | −2.42 ± 0.78 d,A | 16.48 ± 1.43 b,B | 18.12 ± 1.64 c,A |
| ACT-2.5 | 52.33 ± 1.56 b,B | 57.07 ± 1.64 b,A | 11.70 ± 1.78 a,A | 5.69 ± 0.59 c,B | 22.07 ± 1.80 a,B | 27.63 ± 1.62 a,A |
| ACT-7.5 | 43.02 ± 1.04 c,B | 46.76 ± 1.70 c,A | 14.14 ± 1.95 a,A | 9.15 ± 0.68 a,B | 17.32 ± 1.41 b,B | 22.41 ± 1.11 b,A |
| ACT-12.5 | 41.73 ± 0.39 c,A | 42.50 ± 1.66 d,A | 14.45 ± 0.73 a,A | 7.89 ± 0.58 b,B | 15.29 ± 0.81 b,A | 16.42 ± 1.32 c,A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dilucia, F.; Lacivita, V.; Nobile, M.A.D.; Conte, A. Improving the Storability of Cod Fish-Burgers According to the Zero-Waste Approach. Foods 2021, 10, 1972. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10091972
Dilucia F, Lacivita V, Nobile MAD, Conte A. Improving the Storability of Cod Fish-Burgers According to the Zero-Waste Approach. Foods. 2021; 10(9):1972. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10091972
Chicago/Turabian StyleDilucia, Flavia, Valentina Lacivita, Matteo Alessandro Del Nobile, and Amalia Conte. 2021. "Improving the Storability of Cod Fish-Burgers According to the Zero-Waste Approach" Foods 10, no. 9: 1972. https://0-doi-org.brum.beds.ac.uk/10.3390/foods10091972