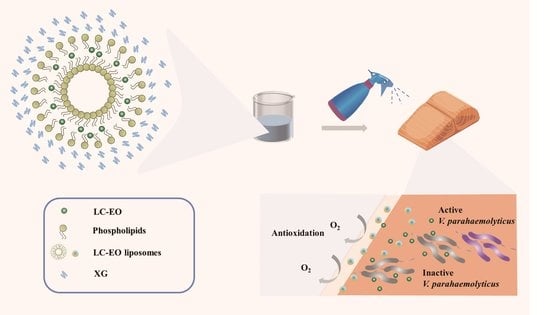
Application of Xanthan-Gum-Based Edible Coating Incorporated with Litsea cubeba Essential Oil Nanoliposomes in Salmon Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Culture
2.2. Antibacterial Activity of LC-EO
2.3. Preparation and Characterization of LC-EO Nanoliposomes
2.3.1. Preparation of LC-EO Nanoliposomes
2.3.2. Characterization of LC-EO Nanoliposomes
Determination of Particle Size, PDI, Zeta Potential, and EE of LC-EO
Fourier Transform Infrared Spectroscopy (FTIR)
SEM
2.4. Preparation of Coating Solutions
2.5. Rheological Properties of the Coating Solutions
2.6. Challenge Test of Edible Coating in Salmon Preservation
2.6.1. Coating Process of Salmon Fillet
2.6.2. pH
2.6.3. Lipid Oxidation
2.6.4. Protein Oxidation
2.6.5. Surface Color
2.6.6. Texture Evaluation
2.6.7. Microbial Analyses
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antibacterial Activity of LC-EO against V. parahaemolyticus
3.2. Characterization of LC-EO Liposomes
3.3. Rheological Analysis of Coating Solutions
3.4. Salmon Preservation by Edible Coating
3.4.1. pH
3.4.2. TBARS Determination
3.4.3. Protein Oxidation
3.4.4. Surface Color
3.4.5. Texture Properties
3.4.6. Microbial Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weichselbaum, E.; Coe, S.; Buttriss, J.; Stanner, S. Fish in the diet: A Review: Fish in the diet. Nutr. Bull. 2013, 38, 128–177. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, J.; Wei, L.; Zhang, Y.; Deng, H.; Guo, Y.; Hu, C.; Meng, Y. Decrease of microbial community diversity, biogenic amines formation, and lipid oxidation by phloretin in Atlantic salmon fillets. LWT 2019, 101, 419–426. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio Spp. infections. Nat. Rev. Dis. Primer. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Santhakumari, S.; Nilofernisha, N.M.; Ponraj, J.G.; Pandian, S.K.; Ravi, A.V. In vitro and in vivo exploration of palmitic acid from Synechococcus elongatus as an antibiofilm agent on the survival of Artemia franciscana against virulent vibrios. J. Invertebr. Pathol. 2017, 150, 21–31. [Google Scholar] [CrossRef]
- Xie, T.; Wu, Q.; Zhang, J.; Xu, X.; Cheng, J. Comparison of Vibrio parahaemolyticus isolates from aquatic products and clinical by antibiotic susceptibility, virulence, and molecular characterisation. Food Control 2017, 71, 315–321. [Google Scholar] [CrossRef]
- Cao, J.; Liu, H.; Wang, Y.; He, X.; Jiang, H.; Yao, J.; Xia, F.; Zhao, Y.; Chen, X. Antimicrobial and antivirulence efficacies of citral against foodborne pathogen Vibrio parahaemolyticus RIMD2210633. Food Control 2021, 120, 107507. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hlima, H.; Tavares, L.; Ennouri, K.; Ben Braiek, O.; Mellouli, L.; Abdelkafi, S.; Mousavi Khaneghah, A. Application of essential oils in meat packaging: A systemic review of recent literature. Food Control 2022, 132, 108566. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Medicinal Plants: Ethno-Uses to Biotechnology Era. In Biotechnology and Production of Anti-Cancer Compounds; Malik, S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–38. [Google Scholar]
- Hu, W.; Li, C.; Dai, J.; Cui, H.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Cui, H.; Lin, L. Encapsulation strategies to enhance the antibacterial properties of essential oils in food system. Food Control 2021, 123, 107856. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef] [Green Version]
- Al-Sahlany, S. Production of Biodegradable Film from Soy Protein and Essential Oil of Lemon Peel and Use It as Cheese Preservative. Basrah J. Agric. Sci. 2017, 30, 27–35. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A Review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Mukherjee, A.; Dutta, J. An Overview of Natural Biopolymers in Food Packaging. In Biopolymer-Based Food Packaging; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 1–28. [Google Scholar]
- Chen, X.; Chen, W.; Lu, X.; Mao, Y.; Luo, X.; Liu, G.; Zhu, L.; Zhang, Y. Effect of chitosan coating incorporated with oregano or cinnamon essential oil on the bacterial diversity and shelf life of roast duck in modified atmosphere packaging. Food Res. Int. 2021, 147, 110491. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT-Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Cui, H.; Ma, C.; Li, C.; Lin, L. Enhancing the antibacterial activity of thyme oil against Salmonella on eggshell by plasma-assisted process. Food Control 2016, 70, 183–190. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antimicrobial mechanism of clove oil on Listeria monocytogenes. Food Control 2018, 94, 140–146. [Google Scholar] [CrossRef]
- Fan, Q.; Yuan, Y.; Zhang, T.; Song, W.; Sheng, Q.; Yue, T. Inhibitory effects of lactobionic acid on Vibrio parahaemolyticus planktonic cells and biofilms. Food Microbiol. 2022, 103, 103963. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, W.; Li, C.; Lin, L. Intelligent release of cinnamon oil from engineered proteoliposome via stimulation of Bacillus Cereus protease. Food Control 2016, 67, 68–74. [Google Scholar] [CrossRef]
- Dag, D.; Guner, S.; Oztop, M.H. Physicochemical mechanisms of different biopolymers’ (lysozyme, gum arabic, whey protein, chitosan) adsorption on green tea extract loaded liposomes. Int. J. Biol. Macromol. 2019, 138, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lu, J.; Li, C.; Lin, L. Fabrication of Phospholipid nanofibers containing eugenol@cationic starch nanoparticles against Bacillus Cereus in Beef. LWT 2021, 144, 111262. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Yang, H. Effects of sucrose addition on the rheology and microstructure of κ-carrageenan gel. Food Hydrocoll. 2018, 75, 164–173. [Google Scholar] [CrossRef]
- Cai, M.; Zhong, H.; Li, C.; Aliakbarlu, J.; Zhang, H.; Cui, H.; Lin, L. Application of composite coating of Nostoc commune Vauch polysaccharides and sodium carboxymethyl cellulose for preservation of salmon fillets. Int. J. Biol. Macromol. 2022, 210, 394–402. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Hossain, M.N.; Leonard, W.; Fang, Z. Effect of sorghum bran incorporation on the physicochemical and microbial properties of beef sausage during cold storage. Food Control 2022, 132, 108544. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Dong, Q.; Li, L. Fabrication of antibacterial fibrous films by electrospinning and their application for Japanese sea bass (Lateolabrax japonicus) preservation. LWT 2021, 149, 111870. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Xu, M.; Lian, Z.; Chen, X.; Yao, X.; Lu, C.; Niu, X.; Xu, M.; Zhu, Q. Effects of resveratrol on lipid and protein co-oxidation in fish oil-enriched whey protein isolate emulsions. Food Chem. 2021, 365, 130525. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, G.; Li, C.; Chen, X.; Cui, H.; Lin, L. Pleurotus eryngii polysaccharide nanofiber containing pomegranate peel polyphenol/chitosan nanoparticles for control of E. coli O157:H7. Int. J. Biol. Macromol. 2021, 192, 939–949. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Inhibition of Escherichia Coli O157:H7 biofilm on vegetable surface by solid liposomes of clove oil. LWT 2020, 117, 108656. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Li, C.; Chen, X.; Cui, H. Fabrication of a dual-response intelligent antibacterial nanofiber and its application in beef preservation. LWT 2022, 154, 112606. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.-E.; Benoit, J.-P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Bai, M.; Li, C.; Cui, H.; Lin, L. Preparation of self-assembling Litsea cubeba Essential Oil/diphenylalanine peptide micro/nanotubes with enhanced antibacterial properties against Staphylococcus aureus biofilm. LWT 2021, 146, 111394. [Google Scholar] [CrossRef]
- Tian, H.; Lu, Z.; Li, D.; Hu, J. Preparation and characterization of citral-loaded solid lipid nanoparticles. Food Chem. 2018, 248, 78–85. [Google Scholar] [CrossRef]
- Lopez-Polo, J.; Silva-Weiss, A.; Zamorano, M.; Osorio, F.A. Humectability and physical properties of hydroxypropyl methylcellulose coatings with liposome-cellulose nanofibers: Food application. Carbohydr. Polym. 2020, 231, 115702. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Use of xanthan gum for whole cell immobilization and its impact in bioremediation-a review. Bioresour. Technol. 2022, 351, 126918. [Google Scholar] [CrossRef]
- Du, H.; Hu, Q.; Yang, W.; Pei, F.; Kimatu, B.M.; Ma, N.; Fang, Y.; Cao, C.; Zhao, L. Development, physiochemical characterization and forming mechanism of Flammulina velutipes polysaccharide-based edible films. Carbohydr. Polym. 2016, 152, 214–221. [Google Scholar] [CrossRef]
- Stephens, J.W.; Dikeman, M.E.; Unruh, J.A.; Haub, M.D.; Tokach, M.D. Effects of pre-rigor injection of sodium citrate or acetate, or post-rigor injection of phosphate plus salt on post-mortem glycolysis, pH, and pork quality attributes. Meat Sci. 2006, 74, 727–737. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Gao, Y.; Ye, C.; Wang, H. Effect of collagen-lysozyme coating on fresh-salmon fillets preservation. LWT 2017, 75, 59–64. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Zając, M.; Guzik, P.; Tkaczewska, J. The effect of furcellaran-gelatin edible coatings with green and pu-erh tea extracts on the microbiological, physicochemical and sensory changes of salmon sushi stored at 4 °C. Food Control 2019, 100, 83–91. [Google Scholar] [CrossRef]
- Karoui, R.; Hassoun, A. Efficiency of Rosemary and Basil Essential Oils on the Shelf-Life Extension of Atlantic Mackerel (Scomber scombrus) Fillets Stored at 2 °C. J. AOAC Int. 2017, 100, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Jongberg, S.; Racanicci, A.M.C.; Skibsted, L.H. Mate extract is superior to green tea extract in the protection against chicken meat protein thiol oxidation. Food Chem. 2019, 300, 125134. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Zhao, L.; Chen, L.; He, Y.; Yang, H. Vacuum impregnation of fish gelatin combined with grape seed extract inhibits protein oxidation and degradation of chilled tilapia fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef]
- Jin, S.; Wang, M.; Yang, H.; Shan, A.; Feng, X. Dietary supplementation of resveratrol improved the oxidative stability and spatial conformation of myofibrillar protein in frozen-thawed duck breast meat. Food Biosci. 2021, 43, 101261. [Google Scholar] [CrossRef]
- Ottestad, S.; Sørheim, O.; Heia, K.; Skaret, J.; Wold, J.P. Effects of Storage Atmosphere and Heme State on the Color and Visible Reflectance Spectra of Salmon (Salmo salar) Fillets. J. Agric. Food Chem. 2011, 59, 7825–7831. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/ΒCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef]
- Lu, F.; Ding, Y.; Ye, X.; Liu, D. Cinnamon and Nisin in alginate–calcium coating maintain quality of fresh northern snakehead fish fillets. LWT-Food Sci. Technol. 2010, 43, 1331–1335. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Jin, W.; Li, L. Effects of tartary buckwheat polysaccharide combined with nisin edible coating on the storage quality of tilapia (Oreochromis niloticus) fillets: Storage quality of tilapia (Oreochromis niloticus) fillets. J. Sci. Food Agric. 2017, 98, 2880–2888. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva–Rodríguez, S.J. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef]
- Gogoi, R.; Loying, R.; Sarma, N.; Munda, S.; Kumar Pandey, S.; Lal, M. A comparative study on antioxidant, anti-Inflammatory, genotoxicity, anti-microbial activities and chemical composition of fruit and leaf essential oils of Litsea cubeba Pers from North-east India. Ind. Crops Prod. 2018, 125, 131–139. [Google Scholar] [CrossRef]
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wang, Z.-C.; Guo, Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019, 124, 1156–1166. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A.; Khazaei, N. Use of quince seed mucilage edible films containing natural preservatives to enhance physico-chemical quality of rainbow trout fillets during cold storage. Food Sci. Hum. Wellness 2014, 3, 65–72. [Google Scholar] [CrossRef] [Green Version]
- ICMSF. Fish and Seafood Products. In Microorganisms in Foods 8: Use of Data for Assessing Process Control and Product Acceptance, 2nd ed.; Swanson, K.M., Ed.; Springer: New York, NY, USA, 2011; pp. 107–133. [Google Scholar]









| Parameter | Control | 4 mg/mL | 5 mg/mL | 6 mg/mL | 7 mg/mL |
|---|---|---|---|---|---|
| Particle Size (nm) | 101.27 ± 9.51 c | 149.92 ± 9.42 b | 168.10 ± 11.97 ab | 174.22 ± 10.96 a | 185.39 ± 13.46 a |
| PDI | 0.208 ± 0.015 c | 0.232 ± 0.021 abc | 0.250 ± 0.025 ab | 0.254 ± 0.011 a | 0.217 ± 0.022 bc |
| Zeta Potential (mV) | −19.36 ± 0.71 d | −29.28 ± 0.75 b | −32.14 ± 0.52 a | −28.02 ± 0.23 c | −27.07 ± 1.05 c |
| Encapsulation Efficiency (%) | / | 34.2 ± 1.92 b | 37.8 ± 1.58 a | 31.42 ± 1.96 b | 26.60 ± 2.14 c |
| Sample | K (Pa·s) | n | R2 |
|---|---|---|---|
| XG | 6.351 ± 0.073 a | 0.122 ± 0.003 f | 0.9655 |
| 1:1 | 0.730 ± 0.006 b | 0.238 ± 0.002 e | 0.9971 |
| 1:2 | 0.302 ± 0.004 c | 0.298 ± 0.004 d | 0.9951 |
| 1:3 | 0.283 ± 0.002 d | 0.383 ± 0.002 c | 0.9995 |
| 1:4 | 0.204 ± 0.001 e | 0.428 ± 0.001 a | 0.9997 |
| 1:5 | 0.077 ± 0.003 f | 0.415 ± 0.010 b | 0.9842 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Yang, M.; Shi, C.; Li, C.; Lin, L. Application of Xanthan-Gum-Based Edible Coating Incorporated with Litsea cubeba Essential Oil Nanoliposomes in Salmon Preservation. Foods 2022, 11, 1535. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111535
Cui H, Yang M, Shi C, Li C, Lin L. Application of Xanthan-Gum-Based Edible Coating Incorporated with Litsea cubeba Essential Oil Nanoliposomes in Salmon Preservation. Foods. 2022; 11(11):1535. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111535
Chicago/Turabian StyleCui, Haiying, Mei Yang, Ce Shi, Changzhu Li, and Lin Lin. 2022. "Application of Xanthan-Gum-Based Edible Coating Incorporated with Litsea cubeba Essential Oil Nanoliposomes in Salmon Preservation" Foods 11, no. 11: 1535. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111535