Interaction between Gelatin and Mulberry Leaf Polysaccharides in Miscible System: Physicochemical Characteristics and Rheological Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Gelatin–Polysaccharide Miscible Solution
2.3. Phase Diagram
2.4. Measurement of Zeta Potential, Particle Size, and Distribution
2.5. Determination of Rheological Properties
2.5.1. Effect of Concentration, pH, Na+ Concentration, and Temperature on Apparent Viscosity of G-MLPs
2.5.2. Effect of G-MLPs on Viscoelasticity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Phase Diagram of Gelatin–Mulberry Leaf Polysaccharides (G-MLPs) Miscible System
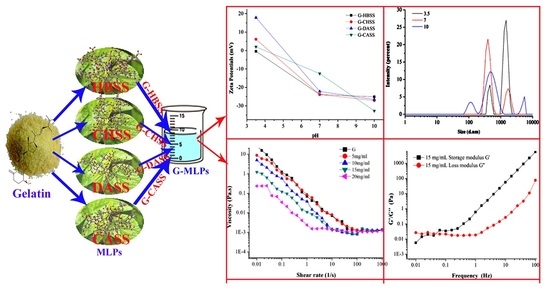
3.2. Zeta Potential Analysis of Gelatin–Mulberry Leaf Polysaccharides (G-MLPs) Miscible System
3.3. Particle Size Analysis of Gelatin–Mulberry Leaf Polysaccharides (G-MLPs) Miscible System
3.4. Rheological Property of Gelatin–Mulberry Leaf Polysaccharide (G-MLPs) Miscible System
3.4.1. Effect of Concentration on Apparent Viscosity of G-MLPs
3.4.2. Effect of pH on Apparent Viscosity of G-MLPs
3.4.3. Effects of Na+ Concentration on Apparent Viscosity of G-MLPs
3.4.4. Effect of System Temperature on Apparent Viscosity of G-MLPs
3.4.5. Effect of G-MLPs on Viscoelasticity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, W.; Xia, Z.; Liu, C.; Ma, S.; Liu, S.; Wu, Y.; Zhu, B.; Xu, C.; Zhao, A. Ionomics, transcriptomics and untargeted metabolomics analyses provide new insights into the Cd response and accumulation mechanisms of mulberry. Environ. Exp. Bot. 2022, 196, 104821. [Google Scholar] [CrossRef]
- Ma, G.Q.; Chai, X.Y.; Hou, G.G.; Zhao, F.L.; Meng, Q.G. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Zeng, J.; Zheng, L.; Zhou, Y.; Xu, K.; Politova, E.D.; Li, G. Effect of rhombohedral-orthorhombic phase transition on structure and electrical properties of KNN-based lead-free piezoceramics. Ferroelectrics 2016, 505, 10–16. [Google Scholar] [CrossRef]
- Eruygur, N.; Dural, E. Determination of 1-Deoxynojirimycin by a developed and validated HPLC-FLD method and assessment of In-vitro antioxidant, α-Amylase and α-Glucosidase inhibitory activity in mulberry varieties from Turkey. Phytomedicine 2019, 53, 234–242. [Google Scholar] [CrossRef]
- Joh, B.; Jeon, E.S.; Lim, S.H.; Park, Y.L.; Park, W.; Chae, H. Intercultural Usage of Mori Folium: Comparison Review from a Korean Medical Perspective. Evid. Based Complementary Altern. Med. 2015, 2015, 379268. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Tangah, J.; Inoue, T.; Chan, H.T. Phenolic constituents and anticancer properties of Morus alba (white mulberry) leaves. J. Integr. Med. 2020, 18, 189–195. [Google Scholar] [CrossRef]
- Qadir, R.; Anwar, F.; Gilani, M.A.; Zahoor, S.; Misbah ur Rehman, M.; Mustaqeem, M. RSM/ANN based optimized recovery of phenolics from mulberry leaves by enzyme-assisted extraction. Czech. J. Food Sci. 2019, 37, 99–105. [Google Scholar] [CrossRef]
- Hu, X.Q.; Jiang, L.; Zhang, J.G.; Deng, W.; Wang, H.L.; Wei, Z.J. Quantitative determination of 1-deoxynojirimycin in mulberry leaves from 132 varieties. Ind. Crops Prod. 2013, 49, 782–784. [Google Scholar] [CrossRef]
- Li, R.L.; Xue, Z.H.; Jia, Y.N.; Wang, Y.J.; Li, S.Q.; Zhou, J.N.; Liu, J.Y.; Zhang, M.; He, C.W.; Chen, H.X. Polysaccharides from mulberry (Morus alba L.) leaf prevents obesity by inhibiting pancreatic lipase in high-fat diet induced mice. Int. J. Biol. Macromol. 2021, 92, 452–460. [Google Scholar] [CrossRef]
- Liao, B.Y.; Hu, H.M.; Thakur, K.; Chen, G.H.; Li, L.; Wei, Z.J. Hypoglycemic activity and the composition analysis of the polysaccharide extracted from the fruit of Mori multicaulis. Curr. Top. Nutraceut. Res. 2018, 16, 1–8. [Google Scholar]
- Hu, X.Q.; Thakur, K.; Chen, G.H.; Hu, F.; Zhang, J.G.; Zhang, H.B.; Wei, Z.J. Metabolic Effect of 1 Deoxynojirimycin from Mulberry Leaves on db/db Diabetic Mice Using Liquid Chromatography−Mass Spectrometry Based Metabolomics. J. Agric. Food Chem. 2017, 65, 4658–4667. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Thakur, K.; Wang, J.; Wang, H.; Hu, F.; Zhang, J.G.; Wei, Z.J. Molecular mechanism of anti-cancerous potential of Morin extracted from mulberry in Hela cells. Food Chem. Toxicol. 2018, 112, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Zhang, Y.Y.; Mocan, A.; Zhang, F.; Zhang, J.G.; Wei, Z.J. 1-Deoxynojirimycin, its potential for management of non-communicable metabolic diseases. Trends Food Sci. Technol. 2019, 89, 88–99. [Google Scholar] [CrossRef]
- Li, L.; Thakur, K.; Cao, Y.Y.; Liao, B.Y.; Zhang, J.G.; Wei, Z.J. Anticancerous potential of polysaccharides sequential extracted from Polygonatum cyrtonema Hua in Human cervical cancer Hela cells. Int. J. Biol. Macromol. 2020, 148, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, T.S.; Zlenko, D.V.; Kiselev, A.V.; Litvin, A.A.; Stovbun, S.V. Antiviral activity of the high-molecular-weight plant polysaccharides (Panavir). Int. J. Biol. Macromol. 2020, 161, 936–938. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Keshavarz Lelekami, A.; Khedmat, L. Plant/algal polysaccharides extracted by microwave: A review on hypoglycemic, hypolipidemic, prebiotic, and immune-stimulatory effect. Carbohydr. Polym. 2021, 266, 118134. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.H.; Liao, A.M.; Huang, J.H.; Thakur, K.; Li, X.L.; Wei, Z.J. Physicochemical and antioxidant potential of polysaccharides sequentially extracted from Amana edulis. Int. J. Biol. Macromol. 2019, 131, 453–460. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Alba, K.; Sims, I.M.; Kontogiorgos, V. Structure and rheology of pectic polysaccharides from baobab fruit and leaves. Carbohydr. Polym. 2021, 273, 118540. [Google Scholar] [CrossRef]
- Liao, B.Y.; Zhu, D.Y.; Thakur, K.; Li, L.; Zhang, J.G.; Wei, Z.J. Thermal and antioxidant properties of polysaccharides sequentially extracted from mulberry leaves (Morus alba L.). Molecules 2017, 22, 22. [Google Scholar] [CrossRef] [Green Version]
- Liao, B.Y.; Li, L.; Tanase, C.; Thakur, K.; Zhu, D.Y.; Zhang, J.G.; Wei, Z.J. The rheological behavior of polysaccharides from mulberry leaves (Morus alba L.). Agronomy 2020, 9, 10. [Google Scholar] [CrossRef]
- Guo, X.; Chen, M.; Li, Y.; Dai, T.; Shuai, X.; Chen, J.; Liu, C. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, G.; Liu, C.; Fu, J.; Hu, D.; Rong, J.; Yang, X. Recent progress in the preparation, chemical interactions and applications of biocompatible polysaccharide-protein nanogel carriers. Food Res. Int. 2021, 147, 110564. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Pei, Y.; Zheng, Y.; Li, Z.; Liu, J.; Zheng, X.; Tang, K.; Kaplan, D.L. Ethanol-induced coacervation in aqueous gelatin solution for constructing nanospheres and networks: Morphology, dynamics and thermal sensitivity. J. Colloid Interface Sci. 2021, 582, 610–618. [Google Scholar] [CrossRef]
- Azarikia, F.; Abbasi, S. Mechanism of soluble complex formation of milk proteins with native gums (tragacanth and Persian gum). Food Hydrocoll. 2016, 59, 35–44. [Google Scholar] [CrossRef]
- Esteban, P.P.; Jenkins, A.T.; Arnot, T.C. Elucidation of the mechanisms of action of Bacteriophage K/nano-emulsion formulations against S. aureus via measurement of particle size and zeta potential. Colloid Surf. B 2016, 139, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wang, T.; Hu, Y.; Wu, J.; Van der Meeren, P. Designing delivery systems for functional ingredients by protein/polysaccharide interactions. Trends Food Sci. Technol. 2022, 119, 272–287. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, W.; Jia, L.; He, Q. The rheological properties of tara gum (Caesalpinia spinosa). Food Chem. 2015, 168, 366–371. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, G.; Liang, Q.; Cai, W.; Zhang, Q. Rheological and microstructural properties of gelatin B/tara gum hydrogels: Effect of protein/polysaccharide ratio, pH and salt addition. LWT-Food Sci. Technol. 2019, 103, 108–115. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, X.; Ji, L.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Phase behaviors involved in surimi gel system: Effects of phase separation on gelation of myofibrillar protein and kappa-carrageenan. Food Res. Int. 2017, 100, 361–368. [Google Scholar] [CrossRef]
- Nakamura, A.; Ohboshi, H.; Sakai, M.; Nomura, K.; Nishiyama, S.; Ashida, H. Extraction of water-soluble polysaccharides from kidney beans and examination of their protein dispersion and stabilization properties under acidic conditions. Food Res. Int. 2021, 144, 110357. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Rao, C.M. The role of surface charge in the desolvation process of gelatin: Implications in nanoparticle synthesis and modulation of drug release. Int. J. Nanomed. 2017, 12, 795–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, S.M.H.; Emam-Djomeh, Z.; Sabatino, P.; Van der Meeren, P. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Ji, X.; Yin, M.; Hao, L.; Shi, M.; Liu, H.; Liu, Y. Effect of inulin on pasting, thermal, rheological properties and in vitro digestibility of pea starch gel. Int. J. Biol. Macromol. 2021, 193, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhang, H.; Xia, Y.; Ai, L. Effects of tamarind seed polysaccharide on gelatinization, rheological, and structural properties of corn starch with different amylose/amylopectin ratios. Food Hydrocoll. 2020, 105, 105854. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Liang, D.; Hou, Y.; Sun, L.; Guo, Y. Low methoxyl pectin gelation under alkaline conditions and its rheological properties: Using NaOH as a pH regulator. Food Hydrocoll. 2018, 79, 560–571. [Google Scholar] [CrossRef]
- Han, W.Y.; Meng, Y.H.; Hu, C.Y.; Dong, G.R.; Qu, Y.L.; Deng, H.; Guo, Y.R. Mathematical model of Ca2+ concentration, pH, pectin concentration and soluble solids (sucrose) on the gelation of low methoxyl pectin. Food Hydrocoll. 2017, 66, 36–48. [Google Scholar] [CrossRef]
- Pan, M.K.; Zhou, F.F.; Liu, Y.; Wang, J.-H. Na+-induced gelation of a low-methoxyl pectin extracted from Premna microphylla Turcz. Food Hydrocoll. 2021, 110, 106153. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Confectionery gels: Gelling behavior and gel properties of gelatin in concentrated sugar solutions. Food Hydrocoll. 2022, 124, 107132. [Google Scholar] [CrossRef]
- Ghosh, A.; Majumdar, A.; Singh Butola, B. Modulating the rheological response of shear thickening fluids by variation in molecular weight of carrier fluid and its correlation with impact resistance of treated p-aramid fabrics. Polym. Test. 2020, 91, 106830. [Google Scholar] [CrossRef]
- Ali Razavi, S.M.; Alghooneh, A.; Behrouzian, F.; Cui, S.W. Investigation of the interaction between sage seed gum and guar gum: Steady and dynamic shear rheology. Food Hydrocoll. 2016, 60, 67–76. [Google Scholar] [CrossRef]
- Chen, L.; Dai, Y.; Hou, H.; Wang, W.; Ding, X.; Zhang, H.; Li, X.; Dong, H. Effect of high pressure microfluidization on the morphology, structure and rheology of sweet potato starch. Food Hydrocoll. 2021, 115, 106606. [Google Scholar] [CrossRef]
- Gharanjig, H.; Gharanjig, K.; Hosseinnezhad, M.; Jafari, S.M. Development and optimization of complex coacervates based on zedo gum, cress seed gum and gelatin. Int. J. Biol. Macromol. 2020, 148, 31–40. [Google Scholar] [CrossRef]
- Cui, T.; Wu, Y.; Ni, C.; Sun, Y.; Cheng, J. Rheology and texture analysis of gelatin/dialdehyde starch hydrogel carriers for curcumin controlled release. Carbohydr. Polym. 2022, 283, 119154. [Google Scholar] [CrossRef] [PubMed]









| Samples | pH | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 3.5 | 4 | 4.5 | 5 | 5.5 | 6 | 7 | 7.5 | 8 | 8.5 | 9 | 9.5 | 10 | |
| G-HBSS | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ■ | ■ | ■ |
| G-CHSS | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ■ | ■ | ■ |
| G-DASS | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ■ | ■ | ■ | ● | ● | ● |
| G-CASS | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ■ | ■ | ■ | ● | ● | ● |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-X.; Liao, B.-Y.; Guan, Z.-J.; Thakur, K.; Khan, M.R.; Busquets, R.; Zhang, J.-G.; Wei, Z.-J. Interaction between Gelatin and Mulberry Leaf Polysaccharides in Miscible System: Physicochemical Characteristics and Rheological Behavior. Foods 2022, 11, 1571. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111571
Zhang X-X, Liao B-Y, Guan Z-J, Thakur K, Khan MR, Busquets R, Zhang J-G, Wei Z-J. Interaction between Gelatin and Mulberry Leaf Polysaccharides in Miscible System: Physicochemical Characteristics and Rheological Behavior. Foods. 2022; 11(11):1571. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111571
Chicago/Turabian StyleZhang, Xiu-Xiu, Bu-Yan Liao, Zi-Jing Guan, Kiran Thakur, Mohammad Rizwan Khan, Rosa Busquets, Jian-Guo Zhang, and Zhao-Jun Wei. 2022. "Interaction between Gelatin and Mulberry Leaf Polysaccharides in Miscible System: Physicochemical Characteristics and Rheological Behavior" Foods 11, no. 11: 1571. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11111571