Valorizing Coffee Silverskin Based on Its Phytochemicals and Antidiabetic Potential: From Lab to a Pilot Scale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Samples
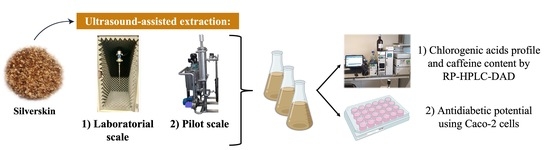
2.3. Extracts Preparation by Ultrasound-Assisted Extraction
2.3.1. Laboratorial Scale
2.3.2. Pilot Scale
2.4. Chlorogenic Acids Profile and Caffeine Content by RP-HPLC-DAD
2.5. Cellular Assays
2.5.1. Caco-2 Cell Culture
2.5.2. Extracts and Standards Preparation
2.5.3. Quantification of 3H-Deoxy-D-Glucose (3H-DG) and 14C-Fructose (14C-FRU) Uptake by Caco-2 Cells
2.5.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5.5. Determination of Cell Viability and Culture Mass
2.5.6. Total Protein Determination
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of the Extracts
3.2. Effect of the Different Extracts on 3H-DG and 14C-FRU Uptake
3.3. Effect of Caffeine and 5-CQA on 3H-DG and 14C-FRU Uptake
3.4. Effect of the Different Extracts upon SGLT1, GLUT2, and GLUT5 mRNA Levels
3.5. Effect of the Different Extracts on Cell Viability and Culture Mass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 14C-FRU | 14C-D-Fructose |
| 3H-DG | [1,2-3H(N)]-deoxy-D-glucose, 3H-deoxy-D-glucose |
| AT | Annealing temperature |
| cDNA | Complementary deoxyribonucleic acid |
| CGAs | Chlorogenic acids |
| CGLs | Chlorogenic acid lactones |
| CQA | Caffeoylquinic acid |
| DM2 | Type 2 diabetes mellitus |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| EDTA | Ethylenediamine tetra-acetic acid |
| FQA | Feruloylquinic acid |
| GLUT | Facilitative glucose transporter |
| HEPES | N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid |
| HFCS | High-fructose corn syrup |
| HPLC | High-performance liquid chromatography |
| LDH | Lactate dehydrogrenase |
| MEM | Minimum essential medium |
| MetS | Metabolic syndrome |
| MMM | Multi-frequency multimode modulation |
| mRNA | Messenger ribonucleic acid |
| NADH | Nicotinamide adenine dinucleotide |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
| RP-HPLC-DAD | Reverse-phase high-performance liquid chromatography coupled to diode array detector |
| RNA | Ribonucleic acid |
| SEM | Standard error of the mean |
| SGLT1 | Sodium-glucose linked transporter 1 |
| SRB | Sulforhodamine B |
| S_LS | Silverskin extract prepared at laboratorial scale |
| S_PS | Silverskin extract prepared at pilot scale |
| S_PS_C | Concentrated silverskin extract prepared at pilot scale |
| UAE | Ultrasound-assisted extraction |
References
- Bessada, S.M.F.; Alves, R.C.; Costa, A.S.G.; Nunes, M.A.; Oliveira, M.B.P.P. Coffea canephora silverskin from different geographical origins: A comparative study. Sci. Total Environ. 2018, 645, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Puga, H.; Alves, R.C.; Costa, A.S.; Vinha, A.F.; Oliveira, M.B.P.P. Multi-frequency multimode modulated technology as a clean, fast, and sustainable process to recover antioxidants from a coffee by-product. J. Clean. Prod. 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Borsetta, G.; Navarini, L.; Abouelenein, D.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G.; Angeloni, S. Coffee silverskin: Characterization of B-vitamins, macronutrients, minerals and phytosterols. Food Chem. 2022, 372, 131188. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; do Prado, F.G.; Pagnoncelli, M.G.B.; Karp, S.G.; Soccol, C.R. Chemical composition and health properties of coffee and coffee by-products. Adv. Food Nutr. Res. 2020, 91, 65–96. [Google Scholar] [CrossRef]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Micheletti, T.O.; Fernandes, R.C.; Nehlig, A. Coffee intake and obesity. In Nutrition in the Prevention and Treatment of Abdominal Obesity, 2nd ed.; Watson, R., Ed.; Academic Press: London, UK, 2019; pp. 329–351. [Google Scholar] [CrossRef]
- Tunnicliffe, J.M.; Cowan, T.; Shearer, J. Chlorogenic acid in whole body and tissue-specific glucose regulation. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 777–785. [Google Scholar] [CrossRef]
- Meng, T.; Antony, B.; Venn, A.; Fraser, B.; Cicuttini, F.; March, L.; Cross, M.; Dwyer, T.; Jones, G.; Laslett, L.L.; et al. Association of glucose homeostasis and metabolic syndrome with knee cartilage defects and cartilage volume in young adults. Semin. Arthritis Rheum. 2020, 50, 192–197. [Google Scholar] [CrossRef]
- De Oliveira, D.T.; Fernandes, I.D.C.; De Sousa, G.G.; Dos Santos, T.A.P.; De Paiva, N.C.N.; Carneiro, C.M.; Evangelista, E.A.; Barboza, N.R.; Guerra-Sá, R. High-sugar diet leads to obesity and metabolic diseases in ad libitum-fed rats irrespective of caloric intake. Arch. Endocrinol. Metab. 2020, 64, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Seo, E.H.; Kim, H.; Kwon, O. Association between total sugar intake and metabolic syndrome in middle-aged Korean men and women. Nutrients 2019, 11, 2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullo, M.; Cozar-Torrell, P.; Salas-Salvado, J. Dietary regulation of glucose metabolism in metabolic syndrome. Curr. Vasc. Pharmacol. 2014, 11, 928–945. [Google Scholar] [CrossRef]
- Hosseini-Esfahani, F.; Bahadoran, Z.; Mirmiran, P.; Hosseinpour-Niazi, S.; Hosseinpanah, F.; Azizi, F. Dietary fructose and risk of metabolic syndrome in adults: Tehran lipid and glucose study. Nutr. Metab. 2011, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Taskinen, M.R.; Packard, C.J.; Borén, J. Dietary fructose and the metabolic syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef] [Green Version]
- Guarino, M.P.; Sacramento, J.; Ribeiro, M.J.; Conde, S.V. Caffeine, insulin resistance, and hypertension. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 747–755. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Lane, J.D. Caffeine, glucose metabolism, and type 2 diabetes. J. Caf. Res. 2011, 1, 23–28. [Google Scholar] [CrossRef]
- Robertson, T.M.; Clifford, M.N.; Penson, S.; Williams, P.; Robertson, M.D. Postprandial glycaemic and lipaemic responses to chronic coffee consumption may be modulated by CYP1A2 polymorphisms. Br. J. Nutr. 2018, 119, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Alves, R.C.; Casal, S.; Oliveira, B. Benefícios do café na saúde: Mito ou realidade? Quím. Nova 2009, 32, 2169–2180. [Google Scholar] [CrossRef]
- Baspinar, B.; Eskici, G.; Ozcelik, A.O. How coffee affects metabolic syndrome and its components. Food Funct. 2017, 8, 2089–2101. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Bondam, A.F.; da Silveira, D.D.; dos Santos, J.P.; Hoffmann, J.F. Phenolic compounds from coffee by-products: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022, 123, 172–186. [Google Scholar] [CrossRef]
- Iriondo-De Hond, A.; Iriondo-De Hond, M.; del Castillo, M.D. Applications of compounds from coffee processing by-products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Rubio, S. Green solvents for the extraction of high added-value compounds from agri-food waste. Food Eng. Rev. 2020, 12, 83–100. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Prot. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Andrade, N.; Silva, C.; Martel, F. The effect of oxidative stress upon intestinal sugar transport: An in vitro study using human intestinal epithelial (Caco-2) cells. Toxicol. Res. 2018, 7, 1236–1246. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. High antioxidant activity of coffee silverskin extracts obtained by the treatment of coffee silverskin with subcritical water. Food Chem. 2012, 135, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Narita, Y.; Inouye, K. Chlorogenic acids from coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 189–199. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; de Paulis, T.; Moreira, D.P.; Trugo, L.C.; Martin, P.R. Chlorogenic acids and lactones in regular and water-decaffeinated arabica coffees. J. Agric. Food Chem. 2006, 54, 374–381. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing By-Products; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 1–26. [Google Scholar] [CrossRef]
- Habtemariam, S. Chemical and pharmacological evidences for coffee as a modulator of type 2 diabetes and metabolic syndrome. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases–The Chemical and Pharmacological Basis of Their Action; Habtemariam, S., Ed.; Academic Press: London, UK, 2019; pp. 793–838. [Google Scholar] [CrossRef]
- Shearer, J.; Farah, A.; de Paulis, T.; Bracy, D.P.; Pencek, R.R.; Graham, T.E.; Wasserman, D.H. Quinides of roasted coffee enhance insulin action in conscious rats. J. Nutr. 2003, 133, 3529–3532. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; Pastoriza, S. Melanoidins in coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2015; pp. 183–188. [Google Scholar] [CrossRef]
- De la Cruz, S.T.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; del Castillo, M.D. An assessment of the bioactivity of coffee silverskin melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Rufián-Henares, J.A. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef] [PubMed]
- Iriondo-DeHond, A.; Fernandez-Gomez, B.; Martinez-Saez, N.; Martirosyan, D.M.; Garcia, M.D.M.; del Castillo, M.D. Coffee silverskin: A low-cost substracte for bioproduction of high-value health promoting products. Ann. Nutr. Food Sci. 2017, 1, 1005. [Google Scholar]
- Nunes, M.A.; Reszczynski, F.; Páscoa, R.N.M.J.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Influence of olive pomace blending on antioxidant activity: Additive, synergistic, and antagonistic effects. Molecules 2021, 26, 169. [Google Scholar] [CrossRef]
- Ontawong, A.; Duangjai, A.; Srimaroeng, C. Coffea arabica bean extract inhibits glucose transport and disaccharidase activity in Caco-2 cells. Biomed. Rep. 2021, 15, 73. [Google Scholar] [CrossRef]
- Welsch, C.A.; Lachance, P.A.; Wasserman, B.P. Dietary phenolic compounds: Inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J. Nutr. 1989, 119, 1698–1704. [Google Scholar] [CrossRef]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [Green Version]
- Kellett, G.L.; Brot-Laroche, E. Apical GLUT2: A major pathway of intestinal sugar absorption. Diabetes 2005, 54, 3056–3062. [Google Scholar] [CrossRef] [Green Version]
- Kellett, G.L.; Brot-Laroche, E.; Mace, O.J.; Leturque, A. Sugar absorption in the intestine: The role of GLUT2. Ann. Rev. Nutr. 2008, 28, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, B.; Gordon, L.; Robinson, W.P.; Desoye, G.; Saffery, R. Glucose as a fetal nutrient: Dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation. J. Nutr. Biochem. 2013, 24, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Shanak, S.; Saad, B.; Zaid, H. Metabolic and epigenetic action mechanisms of antidiabetic medicinal Plants. Evid. Based Complement. Alternat. Med. 2019, 2019, 3583067. [Google Scholar] [CrossRef]
- Gouyon, F.; Caillaud, L.; Carrière, V.; Klein, C.; Dalet, V.; Citadelle, D.; Kellett, G.L.; Thorens, B.; Leturque, A.; Brot-Laroche, E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice. J. Physiol. 2003, 552, 823–832. [Google Scholar] [CrossRef] [PubMed]






| Extract | Caffeine | 5-CQA | 4-CQA | 3-CQA | 5-FQA | 4-FQA |
|---|---|---|---|---|---|---|
| S_LS | 27.73 a ± 0.16 | 1.98 a ± 0.05 | 0.15 b ± 0.04 | 0.31 a ± 0.09 | 1.32 a ± 0.05 | 0.43 a ± 0.04 |
| S_PS | 29.61 a ± 1.76 | 1.04 b ± 0.02 | 0.42 a ± 0.01 | 0.19 ab ± 0.04 | 1.05 ab ± 0.30 | 0.39 a ± 0.04 |
| S_PS_C | 27.33 a ±0.07 | 0.72 c ± 0.01 | 0.14 b ± 0.01 | 0.06 b ± 0.03 | 0.59 b ± 0.01 | 0.37 a ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, J.A.B.; Andrade, N.; Machado, S.; Costa, A.S.G.; Puga, H.; Oliveira, M.B.P.P.; Martel, F.; Alves, R.C. Valorizing Coffee Silverskin Based on Its Phytochemicals and Antidiabetic Potential: From Lab to a Pilot Scale. Foods 2022, 11, 1671. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11121671
Peixoto JAB, Andrade N, Machado S, Costa ASG, Puga H, Oliveira MBPP, Martel F, Alves RC. Valorizing Coffee Silverskin Based on Its Phytochemicals and Antidiabetic Potential: From Lab to a Pilot Scale. Foods. 2022; 11(12):1671. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11121671
Chicago/Turabian StylePeixoto, Juliana A. Barreto, Nelson Andrade, Susana Machado, Anabela S. G. Costa, Helder Puga, Maria Beatriz P. P. Oliveira, Fátima Martel, and Rita C. Alves. 2022. "Valorizing Coffee Silverskin Based on Its Phytochemicals and Antidiabetic Potential: From Lab to a Pilot Scale" Foods 11, no. 12: 1671. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11121671