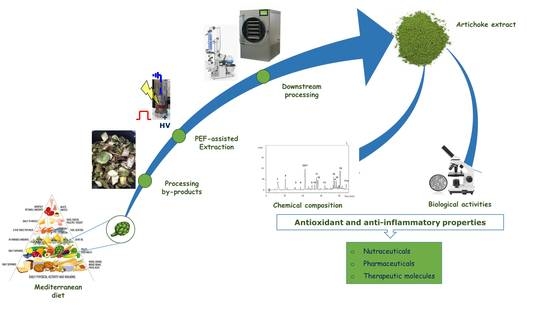
Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. PEF-Assisted Extraction Process
2.3. Characterization of the Extracts
2.3.1. Proximate Analysis of the Extract
2.3.2. Determination of Total Phenolic Content (TPC) and Antioxidant Activity by DPPH and ABTS
2.3.3. HPLC–PDA Analysis of the Extract
2.4. Cell Viability Assay
2.5. Real-Time RT-PCR Assays
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Measurement of Intracellular Reactive Oxygen Species Production
2.8. Statistical Analysis
3. Results
3.1. Proximate Composition of the Extracts
3.2. Total Phenolic Content, Antioxidant Activity and HPLC–PDA Analysis of the Extracts
3.3. Antioxidant Power of Artichoke Extract in LPS-Stimulated Human Macrophages
3.4. Anti-Inflammatory Effects of Artichoke Extract in LPS-Stimulated Human Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McBean, G.A. Integrating science to address food and health within Global Agenda 2030. NPJ Sci. Food 2021, 5, 8. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Martin-Calvo, N. Mediterranean diet and life expectancy; beyond olive oil, fruits, and vegetables. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Vaz-Almeida, M.; Parisi, S. Chemistry of the Mediterranean Diet; Springr: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knuppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; et al. Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071–1090. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food Groups and Risk of Overweight, Obesity, and Weight Gain: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knuppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.M.; Knuppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe Artichoke: A Functional Food and Source of Nutraceutical Ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bohn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [23Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- López-Salas, L.; Borrás-Linares, I.; Quintin, D.; García-Gomez, P.; Giménez-Martínez, R.; Segura-Carretero, A.; Lozano-Sánchez, J. Artichoke By-Products as Natural Source of Phenolic Food Ingredient. Appl. Sci. 2021, 11, 3788. [Google Scholar] [CrossRef]
- Frutos, M.J.; Ruiz-Cano, D.; Valero-Cases, E.; Zamora, S.; Pérez-Llamas, F. Artichoke (Cynara scolymus L.). In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 135–138. [Google Scholar]
- Shallan, M.; Ali, M.; Meshrf, W.; Marrez, D. In vitro antimicrobial, antioxidant and anticancer activities of globe artichoke (Cynara cardunculus var. scolymus L.) bracts and receptacles ethanolic extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar] [CrossRef]
- Fratianni, F.; Pepe, R.; Nazzaro, F. Polyphenol Composition, Antioxidant, Antimicrobial and Quorum Quenching Activity of the “Carciofo di Montoro” (Cynara cardunculus var. scolymus) Global Artichoke of the Campania Region, Southern Italy. Food Nutr. Sci. 2014, 5, 2053–2062. [Google Scholar] [CrossRef] [Green Version]
- Gaafar, A.A.; Salama, Z.A.E.-R. Phenolic Compounds from Artichoke (Cynara scolymus L.) By-products and their Antimicrobial Activities. J. Biol. Agric. Healthc. 2013, 3, 1–6. [Google Scholar]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastro-intestinal digestion. J. Funct. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Phenolic Composition of Artichoke Waste and Its Antioxidant Capacity on Differentiated Caco-2 Cells. Nutrients 2019, 11, 1723. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of artichoke, milk thistle and borututu. Ind. Crops Prod. 2013, 49, 61–65. [Google Scholar] [CrossRef]
- Curadi, M.; Picciarelli, P.; Lorenzi, R.; Graifenberg, A.; Ceccarelli, N. Antioxidant activity and phenolic compounds in the edible parts of early and late Italian artichoke (Cynara scolymus L.) varieties. Ital. J. Food Sci. 2005, 17, 33–44. [Google Scholar]
- Lutz, M.; Henríquez, C.; Escobar, M. Chemical composition and antioxidant properties of mature and baby artichokes (Cynara scolymus L.), raw and cooked. J. Food Compos. Anal. 2011, 24, 49–54. [Google Scholar] [CrossRef]
- Mabeau, S.; Celine, B.; Hélias, A.B.; Chodosas, O.; Surbled, M.; Metra, P.; Durand, D.; Morice, G.; Chesne, C.; Mekideche, K. Antioxidant activity of artichoke extracts and by-products. Acta Hortic. 2007, 730, 491–496. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Dhen, N.; Gargouri, M.; Boulila, A. Chemical Composition, Antioxidant Potential and Enzymes Inhibitory Properties of Globe Artichoke By-Products. Chem. Biodivers. 2020, 17, e2000073. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Caballero, E.; Pérez, E.; Zúñiga, M.E. Effect of extraction conditions on total phenolic content and antioxidant capacity of pretreated wild Peumus boldus leaves from Chile. Food Bioprod. Process. 2014, 92, 328–333. [Google Scholar] [CrossRef]
- Khan, M.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of novel technologies on polyphenols during food processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.C.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2019, 115, 177–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Puértolas, E.; Barba, F.J. Electrotechnologies applied to valorization of by-products from food industry: Main findings, energy and economic cost of their industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds From Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Yu, X.; Gouyo, T.; Grimi, N.; Bals, O.; Vorobiev, E. Pulsed electric field pretreatment of rapeseed green biomass (stems) to enhance pressing and extractives recovery. Bioresour Technol. 2016, 199, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Mena-Garcia, A.; Rodriguez-Sanchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by Microwave Assisted Extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Machado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar] [CrossRef]
- Battipaglia, G.; De Vito, G.; Donsì, F.; Ferrari, G.; Pataro, G. Enhancement of polyphenols extraction from involucral bracts of artichokes. In Proceedings of the International Conference on Bio and Food Electrotechnologies, Universite’ de Technologie de, Compiègne, Compiègne, France, 23 October 2009; pp. 40–44. [Google Scholar]
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of pulsed electric field in the production of juice and extraction of bioactive compounds from blueberry fruits and their by-products. J. Food Sci. Technol. 2015, 52, 5898–5905. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, S.; Ferrari, G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Phenolic Compounds From White Grape Pomace Using Response Surface Methodology. Front. Sustain. Food Syst. 2022, 6, 854968. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of Pulsed Electric Field Treatments for the Enhancement of Mass Transfer from Vegetable Tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Kostas, E.T.; Wilkinson, S.J.; White, D.A.; Cook, D.; Daniel, A. Optimization of a total acid hydrolysis based protocol for the quantification of carbohydrate in macroalgae. J. Algal Biomass Util. 2016, 7, 21–36. [Google Scholar]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding solutions for agricultural wastes: Antioxidant and antitumor properties of pomegranate Akko peel extracts and β-glucan recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, G.; Plastina, P.; Augimeri, G.; Catalano, S.; Giordano, C.; Barone, I.; Morelli, C.; Giordano, F.; Gelsomino, L.; Sisci, D.; et al. Modulating Tumor-Associated Macrophage Polarization by Synthetic and Natural PPARγ Ligands as a Potential Target in Breast Cancer. Cells 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovito, D.; Gionfriddo, G.; Barone, I.; Giordano, C.; Grande, F.; De Amicis, F.; Lanzino, M.; Catalano, S.; Ando, S.; Bonofiglio, D. Ligand-activated PPARgamma downregulates CXCR4 gene expression through a novel identified PPAR response element and inhibits breast cancer progression. Oncotarget 2016, 7, 65109–65124. [Google Scholar] [CrossRef] [Green Version]
- Giordano, C.; Barone, I.; Vircillo, V.; Panza, S.; Malivindi, R.; Gelsomino, L.; Pellegrino, M.; Rago, V.; Mauro, L.; Lanzino, M.; et al. Activated FXR Inhibits Leptin Signaling and Counteracts Tumor-promoting Activities of Cancer-Associated Fibroblasts in Breast Malignancy. Sci. Rep. 2016, 6, 21782. [Google Scholar] [CrossRef]
- Augimeri, G.; Plastina, P.; Gionfriddo, G.; Rovito, D.; Giordano, C.; Fazio, A.; Barone, I.; Catalano, S.; Ando, S.; Bonofiglio, D.; et al. N-Eicosapentaenoyl Dopamine, A Conjugate of Dopamine and Eicosapentaenoic Acid (EPA), Exerts Anti-inflammatory Properties in Mouse and Human Macrophages. Nutrients 2019, 11, 2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noriega-Rodríguez, D.; Soto-Maldonado, C.; Torres-Alarcón, C.; Pastrana-Castro, L.; Weinstein-Oppenheimer, C.; Zúñiga-Hansen, M.E. Valorization of Globe Artichoke (Cynara scolymus) Agro-Industrial Discards, Obtaining an Extract with a Selective Effect on Viability of Cancer Cell Lines. Processes 2020, 8, 715. [Google Scholar] [CrossRef]
- Órbenes, G.; Rodríguez-Seoane, P.; Torres, M.D.; Chamy, R.; Zúñiga, M.E.; Domínguez, H. Valorization of Artichoke Industrial By-Products Using Green Extraction Technologies: Formulation of Hydrogels in Combination with Paulownia Extracts. Molecules 2021, 26, 4386. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- Boubaker, M.; Omri, A.E.; Blecker, C.; Bouzouita, N. Fibre concentrate from artichoke (Cynara scolymus L.) stem by-products: Characterization and application as a bakery product ingredient. Food Sci. Technol. Int. 2016, 22, 759–768. [Google Scholar] [CrossRef]
- Ferioli, F.; D’Antuono, L.F. Phenolic compounds in local Italian types of cultivated cardoon (Cynara cardunculus L. var. altilis DC) stalks and artichoke (Cynara cardunculus L. var. scolymus L.) edible sprouts. J. Food Compos. Anal. 2022, 106, 104342. [Google Scholar] [CrossRef]
- Shuhui, S.; Hongju, H.; Xiaowei, T.; Wenqi, W. Determination of polyphenols and chlorogenic acid in artichoke (Cynara scolymus L.). Acta Hortic. 2010, 856, 167–172. [Google Scholar] [CrossRef]
- Carpentieri, S.; Mazza, L.N.M.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed electric fields- and ultrasound-assisted green extraction of valuable compounds from Origanum vulgare L. and Thymus serpyllum L. Int. J. Food Sci. Technol. 2021, 56, 4834–4842. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.; Brunton, N.; Ferrari, G.; Lyng, J.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Ryan, K.A.; Smith, M.F., Jr.; Sanders, M.K.; Ernst, P.B. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect. Immun. 2004, 72, 2123–2130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidelines for use of nutrition claims. Available online: https://www.foedevarestyrelsen.dk/SiteCollectionDocuments/25_PDF_word_filer%20til%20download/07kontor/Maerkning/Codex%20guidelines%20nutrition%20and%20health%20claims.pdf (accessed on 4 March 2022).
- Antonio, Z. Response surface methodology analysis of polyphenol recovery from artichoke waste. Am. J. Appl. Sci. 2014, 11, 1463. [Google Scholar] [CrossRef] [Green Version]
- Quispe, M.A.; Valenzuela, J.A.P.; Cruz, A.R.H.; Silva, C.R.E.; Quinonez, G.H.; Cervantes, G.M.M. Optimization of ultrasound-assisted extraction of polyphenols from globe artichoke (Cynara scolymus L.) bracts residues using response surface methodology. Acta Sci. Pol. Technol. Aliment. 2021, 20, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Zazzali, I.; Gabilondo, J.; Peixoto Mallmann, L.; Rodrigues, E.; Perullini, M.; Santagapita, P.R. Overall evaluation of artichoke leftovers: Agricultural measurement and bioactive properties assessed after green and low-cost extraction methods. Food Biosci. 2021, 41, 100963. [Google Scholar] [CrossRef]
- Kollia, E.; Markaki, P.; Zoumpoulakis, P.; Proestos, C. Comparison of Different Extraction Methods for the Determination of the Antioxidant and Antifungal Activity of Cynara scolymus and C. cardunculus Extracts and Infusions. Nat. Prod. Commun. 2017, 12, 423–426. [Google Scholar]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Artichoke (Cynara cardunculus L. var. scolymus) waste as a natural source of carbonyl trapping and antiglycative agents. Food Res. Int. 2017, 100, 780–790. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.; Carle, R.; Schieber, A. Identification and Quantification of Caffeoylquinic Acids and Flavonoids from Artichoke (Cynara scolymus L.) Heads, Juice, and Pomace by HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2004, 52, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic Acid Improves Late Diabetes through Adiponectin Receptor Signaling Pathways in db/db Mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef] [Green Version]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.-J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, R.; Feng, C.; Li, X.; Huang, S.; Wang, L.; Liu, Z.; Jiang, J.; Han, Y. Chlorogenic acid inhibits esophageal squamous cell carcinoma growth in vitro and in vivo by downregulating the expression of BMI1 and SOX2. Biomed. Pharmacother. 2020, 121, 109602. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, S.; Stumpf, M.; Strehlow, K.; Schmid, A.; Schieffer, B.; Bohm, M.; Nickenig, G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ. Res. 2004, 94, 534–541. [Google Scholar] [CrossRef] [Green Version]





| Moisture content | 0.17 ± 0.02 |
| Ash content | 3.64 ± 0.21 |
| Protein content | 9.48 ± 0.92 |
| Fat content | 0.42 ± 0.06 |
| Carbohydrates | 66.67 ± 1.24 |
| Total dietary fiber | 19.62 ± 1.21 |
| Peak No. | Compound | Max Absorption Wavelength (nm) | Retention Time (min) | Concentration (mg/gDW) | |
|---|---|---|---|---|---|
| Untreated | PEF-Treated | ||||
| 1 | Gallic acid | 271 | 6.35 | nd | 0.02 ± 0.003 |
| 2 | Catechin | 280 | 14.36 | 0.35 ± 0.03 a | 0.89 ± 0.06 b |
| 3 | Chlorogenic acid | 320 | 14.83 | 4.70 ± 0.37 a | 6.02 ± 0.63 b |
| 4 | Epicatechin | 280 | 16.36 | 0.10 ± 0.01 a | 0.39 ± 0.07 b |
| 5 | Cynarine | 320 | 17.24 | 0.09 ± 0.01 a | 0.15 ± 0.03 b |
| 6 | Sinapic acid | 330 | 20.24 | 0.16 ± 0.02 a | 0.31 ± 0.04 b |
| 7 | Naringin | 283 | 20.93 | 2.06 ± 0.08 a | 3.46 ± 0.36 b |
| 8 | Rutin | 260 | 21.32 | 1.25 ± 0.06 a | 1.96 ± 0.07 b |
| 9 | Phlorizin | 280 | 22.61 | 0.04 ± 0.002 a | 0.20 ± 0.02 b |
| IC50 | ||
|---|---|---|
| Sample | DPPH | ABTS |
| Artichoke extract | 2.4 ± 0.5 mg/mL | 2.0 ± 0.2 mg/mL |
| Positive control | ||
| Trolox | 21.6 ± 2.9 µg/mL | 26.4 ± 2.5 µg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpentieri, S.; Augimeri, G.; Ceramella, J.; Vivacqua, A.; Sinicropi, M.S.; Pataro, G.; Bonofiglio, D.; Ferrari, G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods 2022, 11, 2250. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11152250
Carpentieri S, Augimeri G, Ceramella J, Vivacqua A, Sinicropi MS, Pataro G, Bonofiglio D, Ferrari G. Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages. Foods. 2022; 11(15):2250. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11152250
Chicago/Turabian StyleCarpentieri, Serena, Giuseppina Augimeri, Jessica Ceramella, Adele Vivacqua, Maria Stefania Sinicropi, Gianpiero Pataro, Daniela Bonofiglio, and Giovanna Ferrari. 2022. "Antioxidant and Anti-Inflammatory Effects of Extracts from Pulsed Electric Field-Treated Artichoke By-Products in Lipopolysaccharide-Stimulated Human THP-1 Macrophages" Foods 11, no. 15: 2250. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11152250