Membrane Technology for Valorization of Mango Peel Extracts
Abstract
:1. Introduction
2. Materials and Methods
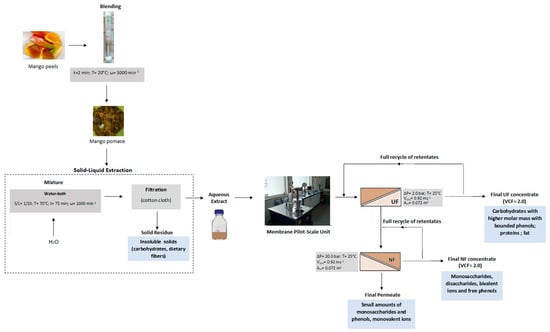
2.1. Pretreatment of Mango Peels and Production of Aqueous Extracts
2.2. Characterization of Mango Peels
2.3. Characterization of Aqueous Extracts
2.4. Molecular Weight of Polysaccharides in UF/NF Fractions
2.5. Membranes and Experimental Set-Up
2.6. Experimental Design
2.6.1. Membrane Cleaning
2.6.2. Determination of Hydraulic Permeability of Membranes to Deionized Water
2.6.3. Ultrafiltration Experiments of Aqueous Extracts
2.6.4. Nanofiltration Experiments of Ultrafiltration Permeates
2.6.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Mango Peels
3.2. Physicochemical Characterization of Aqueous Extracts
3.3. Ultrafiltration of Aqueous Extracts
3.3.1. Ultrafiltration with Total Recirculation
3.3.2. Ultrafiltration in Concentration Mode
3.3.3. Fractionation of Extracts by UF
3.4. Nanofiltration of Ultrafiltration Permeates
3.4.1. Nanofiltration with Total Recirculation and Concentration Modes
3.4.2. Rejection Coefficients of Compounds Fractionated by NF Membranes
3.5. Molecular Weight Distribution of Compounds Separated by UF/NF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- del Pilar Sanchez-Camargo, A.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of mango peel: Proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Altendorf, S. Major Tropical Fruits Market Review 2018; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2019; p. 1. [Google Scholar]
- Shamili, M. The estimation of mango fruit total soluble solids using image processing technique. Sci. Hortic. 2019, 249, 383–389. [Google Scholar] [CrossRef]
- Owino, W.O.; Ambuko, J.L. Mango fruit processing: Options for small-scale processors in developing countries. Open Agric. J. 2021, 11, 1105. [Google Scholar] [CrossRef]
- Bres, P.A.; Beily, M.E.; Young, B.J.; Gasulla, J.; Butti, M.; Crespo, D.; Candal, R.; Komilis, D.P. Performance of semi-continuous anaerobic co-digestion of poultry manure with fruit and vegetable waste and analysis of digestate quality: A bench scale study. J. Waste Manag. 2018, 82, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, H.W.; Philip, L.; Suraishkumar, G.K.; Karthikaichamy, A.; Sen, T.K. Anaerobic co-digestion of activated sludge and fruit and vegetable waste: Evaluation of mixing ratio and impact of hybrid (microwave and hydrogen peroxide) sludge pre- treatment on two-stage digester stability and biogas yield. J. Water Process Eng. 2020, 37, 101498. [Google Scholar] [CrossRef]
- Ajila, C.M.; Bhat, S.G.; Rao, U.J.S.P. Valuable components of raw and ripe peels from two Indian mango varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Umbreen, H.; Arshad, M.U.; Saeed, F.; Bhatty, N.; Hussain, A.I. Probing the functional potential of agro-industrial wastes in dietary interventions. J. Food Process. Preserv. 2015, 39, 1665–1671. [Google Scholar] [CrossRef]
- Gómez, M.; Martinéz, M.M. Fruit and vegetable by-products as novel ingredients to improve the nutritional quality of baked goods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2119–2135. [Google Scholar] [CrossRef]
- Sharma, L.; Saini, C.S.; Sharma, H.K.; Sandhu, K.S. Biocomposite edible coatings based on cross linked-sesame protein and mango puree for the shelf-life stability of fresh-cut mango fruit. J. Food Process Eng. 2019, 42, 12938. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef] [Green Version]
- Marçal, S.; Pintado, M. Mango peels as food ingredient/additive: Nutritional value, processing, safety and applications. Trends Food Sci. Technol. 2021, 114, 472–479. [Google Scholar] [CrossRef]
- Ajila, C.M.; Rao, U.J.S.P. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- López-Cobo, A.; Verardo, V.; Diaz-de-Cerio, E.; Segura-Carretero, A.; Fernández- Gutiérrez, A.; Gómez-Caravaca, A.M. Use of HPLC and GC-QTOF to determine hydrophilic and lipophilic phenols in mango fruit (Mangifera indica L.) and its by-products. Food Res. Int. 2017, 100, 423–434. [Google Scholar] [CrossRef]
- Boskou, D. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Kiokias, S.; Oreopoulou, V. Antioxidant properties of natural carotenoid extracts against the AAPH-initiated oxidation of food emulsions. Innov. Food Sci. Emerg. Technol. 2006, 7, 132–139. [Google Scholar] [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Valette, J.; Guichard, É.; Gunata, Z. Impact of fruit texture on the release and perception of aroma compounds during in vivo consumption using fresh and processed mango fruits. Food Chem. 2018, 239, 806–815. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Uddin, J.; Siddiqui, A.J.; Akram, M.I. Quantification of aroma constituents of mango sap from different Pakistan mango cultivars using gas chromatography triple quadrupole mass spectrometry. Food Chem. 2016, 196, 1355–1360. [Google Scholar] [CrossRef]
- Oliver-Simancas, R.; Muñoz, R.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Mango by-products as a natural source of valuable odor-active compounds. J. Sci. Food Agric. 2020, 100, 4688–4695. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Kabir, F.; Tow, W.W.; Hamauzu, Y.; Katayama, S.; Tanaka, S.; Nakamura, S. Antioxidant and cytoprotective activities of extracts prepared from fruit and vegetable wastes and by-products. Food Chem. 2015, 167, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Donato, L.; Drioli, E. Ultrafiltration of kiwifruit juice: Operating parameters, juice quality and membrane fouling. J. Food Eng. 2007, 79, 613–621. [Google Scholar] [CrossRef]
- Cassano, A.; Jiao, B.; Drioli, E. Production of concentrated kiwifruit juice by integrated membrane process. Food Res. Int. 2004, 37, 139–148. [Google Scholar] [CrossRef]
- Cassano, A.; Castro-Muñoz, R.; Conidi, C.; Drioli, E. Recent developments in membrane technologies for concentration of liquid foods and food ingredients. In Innovative Food Processing Technologies—A Comprehensive Review; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 3, Chapter 6; pp. 100–121. [Google Scholar] [CrossRef]
- Versari, A.; Ferrarini, R.; Parpinello, G.P.; Galassi, S. Concentration of Grape Must by Nanofiltration Membranes. Food Bioprod. Process. 2003, 81, 275–278. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra- and nanofiltration of aqueous extract from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Sicari, V.; Ursino, C.; Manfredi, I.; Cassano, A. Concentration of Bioactive Compounds from Elderberry (Sambucus nigra L.) Juice by Nanofiltration Membranes. Plant Foods Hum. Nutr. 2018, 73, 336–343. [Google Scholar] [CrossRef]
- Makinistian, F.G.; Sette, P.; Gallo, L.; Bucalá, V.; Salvatori, D. Optimized aqueous extracts of maqui (Aristotelia chilensis) suitable for powder production. J. Food Sci. Technol. 2019, 56, 3553–3560. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Tang, B.; Park, H.E.; Row, K.H. Simultaneous extraction of flavonoids from chaecyparis obtuse using deep eutectic solvents as additives of conventional extractions solvents. J. Chromatogr. Sci. 2015, 53, 836–840. [Google Scholar] [CrossRef] [Green Version]
- AOAC Official Methods of Analysis. Solids (total) in fruit and fruit products—Method 920.151. In Official Methods of Analysis of AOAC International; Cunliffe, P., Ed.; AOAC Official Methods of Analysis: Gaithersburg, MD, USA, 1990. [Google Scholar]
- AOAC Official Methods of Analysis. Acidity (titrable) for fruit and fruit products—Method 943.03. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2000; ISBN 0935584544. [Google Scholar]
- AOAC Official Methods of Analysis. Crude protein for fruit products—Method 920.152. In Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2000; ISBN 0935584544. [Google Scholar]
- AOAC Official Methods of Analysis. Crude fat. In Official Methods of Analysis of AOAC International; Cunliffe, P., Ed.; AOAC Official Methods of Analysis: Gaithersburg, MD, USA, 1984. [Google Scholar]
- AOAC Official Methods of Analysis. Ash of fruits and fruit products—Method 940.26. In Official Methods of Analysis of AOAC International; Cunliffe, P., Ed.; AOAC Official Methods of Analysis: Gaithersburg, MD, USA, 1990; p. 915. [Google Scholar]
- AOAC Official Methods of Analysis. Total, Soluble and Insoluble Dietary Fiber in Foods—Method 991.43. In Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Clarification of high-added value products from olive mill wastewater. J. Food Eng. 2010, 99, 190–197. [Google Scholar] [CrossRef]
- Araújo, A.; Freitas, F.; Sevrin, C.; Grandfils, G.; Reis, M.A.M. Co-production of chitin-glucan complex and xylitol by Komagataella pastoris using glucose and xylose mixtures as carbon source. Carbohydr. Polym. 2017, 166, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Al-Duais, M.; Müller, L.; Böhm, V.; Jetschke, G. Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: Use of different assays. Eur. Food Res. Technol. 2009, 228, 813–821. [Google Scholar] [CrossRef]
- Macedo, A.; Monteiro, J.; Duarte, E. A contribution for the valorisation of sheep and goat cheese whey through nanofiltration. Membranes 2018, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Danish Separation Systems. Operating Manual DSS LabUnit M20; Danish Separation Systems: Nakskov, Denmark, 2002. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 418–420. ISBN 978-0792342489. [Google Scholar]
- Jeanson, S.; Floury, J.; Gagnaire, V.; Lortal, S.; Thierry, A. Bacterial colonies in solid media and foods: A review on their growth and interactions with the micro-environment. Front. Microbiol. 2015, 6, 1284. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Chicaybam, G.; Araujo, M.T.; Manhães, L.R.T.; Sabaa-Srur, A.U.O. Centesimal and mineral composition of mango peel and pulp (Mangifera indica L. cv. Tommy Atkins). Rev. Bras. Frutic. 2010, 32, 1206–1210. [Google Scholar] [CrossRef]
- Asp, N.G. Dietary carbohydrates: Classification by chemistry and physiology. Food Chem. 1996, 57, 9–14. [Google Scholar] [CrossRef]
- Parra, V.; Anaguano, M.; Molina, M.; Yerovi, D.; Ruales, J. Characterization and quantification of bioactive compounds and antioxidant activity in three different varieties of mango (Mangifera indica L.) peel from the Ecuadorian region using HPLC-UV/VIS and UPLC-PDA. NFS J. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Imran, M.; Butt, M.S.; Anjum, F.M. Chemical profiling of different mango peel varieties. Pak. J. Nutr. 2013, 12, 934–942. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greibi, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of Tommy Atkins mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef]
- Macedo, A.; Duarte, E.; Pinho, M. The role of concentration polarization in ultrafiltration of ovine cheese whey. J. Membr. Sci. 2011, 381, 34–40. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Macedo, A.; Duarte, E.; Fragoso, R. Assessment of the performance of three ultrafiltration membranes for fractionation of ovine second cheese whey. Int. Dairy J. 2015, 48, 31–37. [Google Scholar] [CrossRef]
- Resende, A.; Catarino, S.; Geraldes, V.; De Pinho, M. Separation and purification by ultrafiltration of white wine high molecular weight polysaccharides. Ind. Eng. Chem. Res. 2013, 52, 8875–8879. [Google Scholar] [CrossRef]
- Evans, P.J.; Bird, M.R.; Pïhlajamäki, A.; Nyström, M. The influence of hydrophobicity, roughness and charge upon ultrafiltration membranes for black tea liquor clarification. J. Membr. Sci. 2008, 313, 250–262. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; González, M. Using drying treatments to stabilise mango peel and seed: Effect on antioxidant activity. LWT—Food Sci. Technol. 2012, 45, 261–268. [Google Scholar] [CrossRef]
- Caravaca, A.M.G.; Lópes-Cobo, A.; Verardo, V.; Carretera, A.S.; Fernández-Gutiérrez, A. HPLC-DAD-q-TOF-MS as a powerful platform for the determination of phenolic and other polar compounds in the edible part of mango and its by-products (peel, seed, and seed husk). Electrophoresis 2015, 37, 7–8. [Google Scholar] [CrossRef]
- Daufin, G.; René, F.; Aimar, P. Les Separations par Membrane dans les Procédés de l´Industrie Alimentaire; Collection Sciences et Techniques Agroalimentaires: Paris, France, 1998. [Google Scholar]
- Oatley-Radcliffe, D.L.; Walters, M.; Ainscough, T.J.; Williams, P.M.; Mohammad, A.W.; Hilal, N. Nanofiltration membranes and processes: A review of research trends over the past decade. J. Water Process. Eng. 2017, 19, 164–171. [Google Scholar] [CrossRef]
- Stoller, M. A three-year long experience of effective fouling inhibition by threshold flux-based optimization methods on a NF membrane module for olive mill wastewater treatment. Membranes 2013, 32, 37–42. [Google Scholar] [CrossRef]
- Macedo, A.; Ochando-Pulido, J.; Fragoso, R.; Duarte, E. The use and performance of nanofiltration membranes for agro-industrial effluents purification. In Nanofiltration; Farrukh, M.A., Ed.; IntechOpen Limited: London, UK, 2018; Chapter 4; pp. 65–84. [Google Scholar] [CrossRef]
- Luo, J.; Shiwei, G.; Wu, Y.; Wan, Y. Separation of sucrose and reducing sugar in cane molasses by nanofiltration. Food Bioprocess Technol. 2018, 11, 913–925. [Google Scholar] [CrossRef]
- Nihal, A.; Türker, G.; Levent, Y. Effect of operating parameters on the separation of sugars by nanofiltration. Sep. Sci. Technol. 1998, 33, 1767–1785. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Ortona, O.; Simões, S.M.N.; Santos, C.I.A.V.; Prazeres, P.M.R.A.; Valente, A.J.M.; Lobo, V.; Burrows, H. Binary mutual diffusion coefficients of aqueous solutions of sucrose, lactose, and fructose in the temperature range from (298.15 to 328.15) K. J. Chem. Eng. Data 2006, 51, 1836–1840. [Google Scholar] [CrossRef]
- Filipou, P.; Mitrouli, S.T.; Vareltzis, P. Sequential Membrane filtration to recover polyphenols and organic acids from red wine lees: The antioxidant properties of the spray-dried concentrate. Membranes 2022, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Luo, S.; Wang, C.; Bai, H.; Lu, J.; Tian, L.; Gao, M.; Wu, J.; Bai, C.; Sun, H. Effects of ultra-high pressure enzyme extraction on characteristics and functional properties of red pitaya (Hylocereus polyrhizus) peel pectic polysaccharides. Food Hydrocoll. 2021, 121, 107016. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; de Almeida, C.P.; Iacomini, M.; Cipriani, T.R.; Cordeiro, L.M.C. Arabinan-rich pectic polysaccharides from buriti (Mauritia flexuosa): An Amazonian edible palm fruit. Carbohydr. Polym. 2015, 122, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Mitrouli, S.T.; Patsios, S.I.; Kazakli, M.; Karabelas, A.J. Valorization of pomegranate husk—Integration of extraction with nanofiltration for concentrated polyphenols recovery. J. Environ. Chem. Eng. 2020, 8, 103951. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [Green Version]



| Membrane | Material b | MWCO a (Da) | Max. Temperature b (°C) | pH Range b | Pressure Range b | Hydraulic Permeability d (Lh−1 m−2 bar−1) |
|---|---|---|---|---|---|---|
| GR60PP (UF) | Polysulfone | 25,000 b | 75 | 2–10 | 1–10 | 63.68 ± 2.94 |
| NF | Polypiperazine | 130 c | 50 | 3–9 | 15–35 | 3.54 ± 0.20 |
| Solution Type | Solution | Time (Min) | Objective |
|---|---|---|---|
| Cleaning | |||
| Alkaline conditions | Sodium hydroxide solution, 0.05% (w/v) | 15 | Removal of organic compounds (proteins, fat, sugars) |
| Na-EDTA a solution, 0.2% (w/v) | 15 | ||
| Acid conditions | Nitric acid solution, 0.25% (w/v) | 15 | Removal of minerals and salts |
| Monohydrate citric acid solution, 0.5% (w/v) | 15 | ||
| Disinfection | Hydrogen peroxide solution, 1000 ppm | 30 | Elimination of microorganisms |
| Samples | ||
|---|---|---|
| Parameter | Mango Peels | Aqueous Extracts (1:10) |
| pH (T = 25 °C) | 4.87 ± 0.03 | 4.12 ± 0.24 |
| Titration acidity (% citric acid) | 0.02 ± 0.001 | - |
| Moisture (% w/w) | 82.29 ± 0.12 | - |
| aw | 0.92 ± 0.01 | - |
| °Bx (total soluble solids) | 15 ± 0.58 | 1.0 ± 0.10 |
| Total protein a (%w/w) | 3.27 ± 0.43 | 9.62 ± 0.20 |
| Fat a (% w/w) | 0.62 ± 0.11 | 0.02 ± 0.01 |
| Ash a (% w/w) | 3.69 ± 0.07 | 12.02 ± 0.83 |
| Raw fiber a (%w/w) | 11.39 ± 0.23 | - |
| Carbohydrates (% w/w) | 81.03 a,b ± 0.05 | 77.48 ± 2.92 |
| Total soluble phenols a (mg EAG/g of mango peel) c | - | 62.5 ± 2.8 |
| Antioxidant capacity a (μmol TE/g of mango peel) | - | 46.1 ± 1.6 (81.6 μmol TE/100 mL) |
| Rejection Coefficients (%) | |||||||
|---|---|---|---|---|---|---|---|
| Total Carbohydrates | Glucose | Galactose | Fructose | Saccharose | Ash | Total Soluble Phenols | Antioxidant Capacity |
| 22.4 ± 2 | 22 ± 3 | 4 ± 1 | 14 ± 2 | 1 ± 0.1 | 2.1 ± 0.1 | 35.0 ± 2 | 75.0 ± 4 |
| Rejection Coefficients (%) | ||||||
|---|---|---|---|---|---|---|
| Total Carbohydrates | Glucose | Fructose | Galactose | Saccharose | Total Soluble Phenols | Antioxidant Capacity |
| 99 ± 2 | 82± 1 | 98 ± 1 | 100 ± 0 | 100 ± 0 | 92± 3 | 99 ± 2 |
| Sample | a Mn (Da) | b Mw (Da) | c PI |
|---|---|---|---|
| Feed (UF) | |||
| Concentrate (UF) | |||
| Permeate (UF) | |||
| Feed (NF) | |||
| Concentrate (NF) | 260 | 355 | 1.37 |
| Permeate (NF) | 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo, A.; Gomes, T.; Ribeiro, C.; Moldão-Martins, M.; Duarte, E.; Alves, V.D. Membrane Technology for Valorization of Mango Peel Extracts. Foods 2022, 11, 2581. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11172581
Macedo A, Gomes T, Ribeiro C, Moldão-Martins M, Duarte E, Alves VD. Membrane Technology for Valorization of Mango Peel Extracts. Foods. 2022; 11(17):2581. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11172581
Chicago/Turabian StyleMacedo, Antónia, Tânia Gomes, Carlos Ribeiro, Margarida Moldão-Martins, Elizabeth Duarte, and Vítor D. Alves. 2022. "Membrane Technology for Valorization of Mango Peel Extracts" Foods 11, no. 17: 2581. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11172581