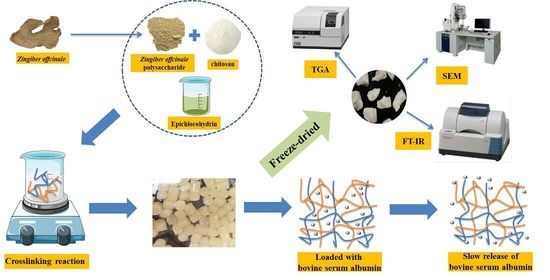
The Synthesis, Characterization, and Protein-Release Properties of Hydrogels Composed of Chitosan-Zingiber offcinale Polysaccharide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Reagents
2.2. Sample Preparation
2.2.1. Extraction of ZOP
2.2.2. Analysis of the Average Molecular Weight (Mw) and Monosaccharide Composition of ZOP
2.2.3. Atomic Force Microscope (AFM) Observation
2.2.4. Preparation of Hydrogels
2.3. Characterization of ZOP/CS Hydrogel
2.3.1. Rheological Measurements
2.3.2. Gel Hardness and Springiness
2.3.3. FT-IR Spectrometric
2.3.4. XRD Analysis
2.3.5. TGA Analysis
2.3.6. SEM Analysis
2.3.7. Water Content of Hydrogels
2.4. Swelling Behavior
2.5. In Vitro Degradation
2.6. In Vitro Release
2.7. Determination of Phagocytic Activity
2.8. Statistical Analysis
3. Results and Analysis
3.1. Extraction of ZOP
3.2. Analysis of the Average Mw and Monosaccharide Composition of ZOP
3.3. AFM Observation of ZOP
3.4. Dynamic Rheological Measurements
3.5. Gel Properties
3.6. FT-IR Spectrometric
3.7. XRD Analysis
3.8. Thermal Properties of the Composed Hydrogels
3.9. Morphology
3.10. Water Content of the Hydrogels
3.11. Swelling Behavior
3.12. In Vitro Degradation
3.13. In Vitro Release
3.14. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darge, H.F.; Andrgie, A.T.; Tsai, H.-C.; Lai, J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, J.; Zhao, D.; Ding, B.; Zhang, L.; Cai, J. High-Flexibility, High-Toughness Double-Cross-Linked Chitin Hydrogels by Sequential Chemical and Physical Cross-Linkings. Adv. Mater. 2016, 28, 5844–5849. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Y.; Zhang, X.; Zhao, X.; Ma, J.; Pu, X.; Wang, Y.; Ran, F.; Wang, Y.; Leng, F.; et al. Snakegourd root/Astragalus polysaccharide hydrogel preparation and application in 3D printing. Int. J. Biol. Macromol. 2019, 121, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Su, T. Research on Design, Synthesis and Application of Novel Polysaccharide Composite Materials. Ph.D. Thesis, Nanjing University of Science and Technology, Nanjing, China, 2020. [Google Scholar]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.-Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef]
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Li, S.H.; Song, B.W.; Zhang, H.L.; Yu, Z.P.; Ma, Y.; Sun, C.Y.; Zhao, C.; Li, J.R. Extraction and antioxidant activity of polysaccharides from ginger. J. Food Saf. Qual. 2017, 8, 1357–1363. [Google Scholar]
- Jafarzadeh, A.; Nemati, M. Therapeutic potentials of ginger for treatment of Multiple sclerosis: A review with emphasis on its immunomodulatory, anti-inflammatory and anti-oxidative properties. J. Neuroimmunol. 2018, 324, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Xia, S.L.; Wu, Q.S. Extraction of ginger polysaccharide and its anti fatigue effect. Jiangsu Agric. Sci. 2014, 42, 240–242. [Google Scholar]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Fookes, F.A.; Mengatto, L.N.; Rigalli, A.; Luna, J.A. Controlled fluoride release for osteoporosis treatment using orally admin-istered chitosan hydrogels. J. Drug Deliv. Sci. Technol. 2019, 51, 268–275. [Google Scholar] [CrossRef]
- Soares, P.A.; Bourbon, A.I.; Vicente, A.A.; Andrade, C.A.; Barros, W.; Correia, M.T.; Pessoa, A.; Carneiro-Da-Cunha, M.G. Development and characterization of hydrogels based on natural polysaccharides: Policaju and chitosan. Mater. Sci. Eng. C 2014, 42, 219–226. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Li, J.; Zhang, Y.; Zhang, R.; Zheng, Y.; Hu, B.; Wu, L.; Zhang, D. Structural characterization and biological activities of a novel polysaccharide from Glehnia littoralis and its application in preparation of nano-silver. Int. J. Biol. Macromol. 2021, 183, 1317–1326. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, R.; Li, L.; Zhang, D.; Liu, Y.; Wu, L.; Zheng, Y. Optimization of Ultrasonic-Assisted Extraction, Characterization, and Antioxidant Activities of Polysaccharides from Sojae Semen Praeparatum. Nat. Prod. Commun. 2021, 16, 1934578X2110206. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Z.; Luo, S.-Y.; Zong, M.-H.; Lou, W.-Y. Multi-functional magnetic hydrogels based on Millettia speciosa Champ residue cellulose and Chitosan: Highly efficient and reusable adsorbent for Congo red and Cu2+ removal. Chem. Eng. J. 2021, 423, 130198. [Google Scholar] [CrossRef]
- Liu, W.; Wang, R.; Li, J.; Xiao, W.; Rong, L.; Yang, J.; Wen, H.; Xie, J. Effects of different hydrocolloids on gelatinization and gels structure of chestnut starch. Food Hydrocoll. 2021, 120, 106925. [Google Scholar] [CrossRef]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 25, 180–188. [Google Scholar] [CrossRef]
- Feki, A.; Hamdi, M.; Jaballi, I.; Zghal, S.; Nasri, M.; Ben Amara, I. Conception and characterization of a multi-sensitive composite chitosan-red marine alga-polysaccharide hydrogels for insulin controlled-release. Carbohydr. Polym. 2020, 236, 116046. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.Y.; Hua, R.H.; Chen, Z.Z.; Lin, X.R.; Zhang, Y.T.; Wu, J.L.; Gao, X.; Li, B. Structural characterization and immunomodu-latory activities of polysaccharides from Suillus granulatus. Sci. Technol. Food. Ind. 2021, 42, 94–103. [Google Scholar] [CrossRef]
- Sun, D.; Yoo, B. Effect of tapioca starch addition on rheological, thermal, and gelling properties of rice starch. LWT—Food Sci. Technol. 2015, 64, 205–211. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, H.; Xia, Y.; Ai, L. Effects of tamarind seed polysaccharide on gelatinization, rheological, and structural properties of corn starch with different amylose/amylopectin ratios. Food Hydrocoll. 2020, 105, 105854. [Google Scholar] [CrossRef]
- von Borries-Medrano, E.; Jaime-Fonseca, M.R.; Aguilar-Méndez, M.A. Tapioca starch-galactomannan systems: Comparative studies of rheological and textural properties. Int. J. Biol. Macromol. 2019, 122, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Yadav, M.P.; Chen, H.; Liu, Y.; Tatsumi, E.; Yin, L. Effects of corn fiber gum (CFG) on the pasting and thermal behaviors of maize starch. Carbohydr. Polym. 2015, 115, 246–252. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Miao, S.; Zhang, L.; Zheng, B. Curdlan enhances the structure of myosin gel model. Food Sci. Nutr. 2019, 7, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Wang, B.; Wen, H.; Cui, B.; Zhang, Z.; Kong, X.; Wang, Y. Improvement of the textural characteristics of curdlan gel by the formation of hydrogen bonds with erythritol. Food Hydrocoll. 2021, 117, 106648. [Google Scholar] [CrossRef]
- Lelifajri, R.; Shabrina. J. Preparation of chitosan composite film reinforced with cellulose isolated from oil palm empty fruit bunch and application in cadmium ions removal from aqueous solutions. Carbohydr. Polym. 2017, 170, 226–233. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Xu, M. Study of the cell responses in tantalum carbide nanoparticles-enriched polysaccharide composite hydrogel. Int. J. Biol. Macromol. 2019, 135, 501–511. [Google Scholar] [CrossRef]
- Hu, X.; Feng, L.; Xie, A.; Wei, W.; Wang, S.; Zhang, J.; Dong, W. Synthesis and characterization of a novel hydrogel: Salecan/polyacrylamide semi-IPN hydrogel with a desirable pore structure. J. Mater. Chem. B 2014, 2, 3646–3658. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Preparation of sulfur nanoparticle-incorporated antimicrobial chitosan films. Food Hydrocoll. 2018, 82, 116–123. [Google Scholar] [CrossRef]
- Soubhagya, A.; Moorthi, A.; Prabaharan, M. Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int. J. Biol. Macromol. 2020, 157, 135–145. [Google Scholar] [CrossRef]
- Ahmad, U.; Sohail, M.; Ahmad, M.; Minhas, M.U.; Khan, S.; Hussain, Z.; Kousar, M.; Mohsin, S.; Abbasi, M.; Shah, S.A.; et al. Chitosan based thermosensitive injectable hydrogels for controlled delivery of loxoprofen: Development, characterization and in-vivo evaluation. Int. J. Biol. Macromol. 2019, 129, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Cheng, W.; Ma, Y.; Zhang, Y.; Li, M.; Zheng, Y.; Zhang, D.; Wu, L. Structural Characterization, Antioxidant and Antibacterial Activities of a Novel Polysaccharide from Zingiber officinale and Its Application in Synthesis of Silver Nanoparticles. Front. Nutr. 2022, 9, 917094. [Google Scholar] [CrossRef] [PubMed]
- Chansoria, P.; Asif, S.; Polkoff, K.; Chung, J.; Piedrahita, J.A.; Shirwaiker, R.A. Characterizing the Effects of Synergistic Thermal and Photo-Cross-Linking during Biofabrication on the Structural and Functional Properties of Gelatin Methacryloyl (GelMA) Hydrogels. ACS Biomater. Sci. Eng. 2021, 7, 5175–5188. [Google Scholar] [CrossRef]
- Guo, W.; Lu, C.-H.; Orbach, R.; Wang, F.; Qi, X.-J.; Cecconello, A.; Seliktar, D.; Willner, I. pH-Stimulated DNA Hydrogels Exhibiting Shape-Memory Properties. Adv. Mater. 2015, 27, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Khoshfetrat, A.B.; Khatami, N.; Ahmadian, M.; Rahbarghazi, R. Comparative study of collagen and gelatin in chitosan-based hydrogels for effective wound dressing: Physical properties and fibroblastic cell behavior. Biochem. Biophys. Res. Commun. 2019, 518, 625–631. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, M.; Manzoor, K.; Purwar, R.; Ikram, S. A review on latest innovations in natural gums based hydrogels: Preparations & applications. Int. J. Biol. Macromol. 2019, 136, 870–890. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, X.; Li, C.; Liu, H.; Cheng, Y.; Lu, J.; Zhang, K.; Wang, H. Super-swelling lignin-based biopolymer hydrogels for soil water retention from paper industry waste. Int. J. Biol. Macromol. 2019, 135, 815–820. [Google Scholar] [CrossRef]
- Pellá, M.C.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.V.; Jacobowski, A.C.; Macedo, M.L.; Batista, K.A.; Fernandes, K.F. Immobilization of antimicrobial trypsin inhibitors onto cashew gum polysaccharide/PVA films. Int. J. Biol. Macromol. 2019, 127, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Nagai, N.; Saijo, S.; Kaji, H.; Nishizawa, M.; Abe, T. In situ formation of injectable chitosan-gelatin hydrogels through double crosslinking for sustained intraocular drug delivery. Mater. Sci. Eng. C 2018, 88, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Mishra, S.; Siddiqui, M.; Jha, U.; Singh, D.; Mahajan, G.R. Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohydr. Polym. 2018, 185, 169–178. [Google Scholar] [CrossRef]
- He, Y.; Gou, S.; Zhou, L.; Tang, L.; Liu, T.; Liu, L.; Duan, M. Amidoxime-functionalized polyacrylamide-modified chitosan containing imidazoline groups for effective removal of Cu2+ and Ni2+. Carbohydr. Polym. 2021, 252, 117160. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, S.; Lee, H. Bio-inspired adhesive catechol-conjugated chitosan for biomedical applications: A mini review. Acta Biomater. 2015, 27, 101–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, J.; Tan, T. Salt-, pH- and temperature-responsive semi-interpenetrating polymer network hydrogel based on poly(aspartic acid) and poly(acrylic acid). Polymer 2006, 47, 7702–7710. [Google Scholar] [CrossRef]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Ding, S.; Liu, C.; Ai, J. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.W.; Zhao, L.; Kim, S.Y. Biomineralized biomimetic organic/inorganic hybrid hydrogels based on hyaluronic acid and poloxamer. Carbohydr. Polym. 2015, 126, 130–140. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Zhao, C.; Kong, W.; Dai, Q.; Chen, Q.; Liu, C.; Sun, R. Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Liu, Y.; Wu, D.; Cai, P.; Pan, Y. Structural characterization and immunomodulatory activity of a water soluble polysaccharide isolated from Botrychium ternatum. Carbohydr. Polym. 2017, 171, 136–142. [Google Scholar] [CrossRef] [Green Version]










| Samples | k (Pa sn) | n | R2 | Gel Hardness (g) | Gel Springiness (g) |
|---|---|---|---|---|---|
| ZOP0 | 0.189 ± 0.012 a | 0.025 ± 0.004 a | 0.991 | 500.497 ± 4.738 e | 0.761 ± 0.005 a |
| ZOP30 | 0.170 ± 0.034 b | 0.017 ± 0.007 b | 0.991 | 521.803 ± 7.198 d | 0.692 ± 0.022 b |
| ZOP50 | 0.152 ± 0.008 c | 0.013 ± 0.005 c | 0.990 | 543.696 ± 2.112 c | 0.653 ± 0.096 c |
| ZOP70 | 0.136 ± 0.011 d | 0.024 ± 0.001 a | 0.995 | 576.965 ± 9.695 b | 0.582 ± 0.039 d |
| ZOP100 | 0.112 ± 0.015 e | 0.012 ± 0.006 c | 0.991 | 606.409 ± 3.265 a | 0.550 ± 0.009 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Y.; Zhang, Y.; Cheng, W.; Li, M.; Hu, B.; Zheng, Y.; Zhang, D.; Wu, L. The Synthesis, Characterization, and Protein-Release Properties of Hydrogels Composed of Chitosan-Zingiber offcinale Polysaccharide. Foods 2022, 11, 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11182747
Jing Y, Zhang Y, Cheng W, Li M, Hu B, Zheng Y, Zhang D, Wu L. The Synthesis, Characterization, and Protein-Release Properties of Hydrogels Composed of Chitosan-Zingiber offcinale Polysaccharide. Foods. 2022; 11(18):2747. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11182747
Chicago/Turabian StyleJing, Yongshuai, Yameng Zhang, Wenjing Cheng, Mingsong Li, Beibei Hu, Yuguang Zheng, Danshen Zhang, and Lanfang Wu. 2022. "The Synthesis, Characterization, and Protein-Release Properties of Hydrogels Composed of Chitosan-Zingiber offcinale Polysaccharide" Foods 11, no. 18: 2747. https://0-doi-org.brum.beds.ac.uk/10.3390/foods11182747